
As confidentially submitted to the Securities and Exchange Commission on July 6, 2021 as amendment No. 2 to the Confidential Submission pursuant to the Jumpstart Our Business Startups Act of 2012
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form F-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
ICECURE MEDICAL LTD.
(Exact name of registrant as specified in its charter)
| State of Israel | 3841 | Not Applicable | ||
| (State
or other jurisdiction of incorporation or organization) |
(Primary
Standard Industrial Classification Code Number) |
(I.R.S.
Employer Identification Number) |
Eyal Shamir Chief Executive Officer 7 Ha’Eshel St., PO Box 3163 Caesarea, 3079504 Israel Tel: +972.4.6230333 |
IceCure Medical Inc. 10 W Prospect Street, Suite 401 Nanuet, NY 10954 Tel: +1-888-902-5716 | |
| (Address, including zip code, and telephone number, | (Name, address, including zip code, and telephone | |
| including area code, of registrant’s principal executive offices) | number, including area code, of agent for service) |
Copies to:
| Oded Har-Even, Esq. | Reut Alfiah, Adv. | |
David Huberman, Esq. Sullivan & Worcester LLP 1633 Broadway New York, NY 10019 Tel: 212.660.3000 |
Sullivan & Worcester Israel (Har-Even & Co.) HaArba’a Towers - 28 HaArba’a St. North Tower, 35th floor Tel-Aviv, Israel 6473925 Tel: + 972.74.758.0480 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date hereof.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☒
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards † provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
| † | The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. |
CALCULATION OF REGISTRATION FEE
| Title of each class of securities to be registered | Amount to be registered(1) | Proposed maximum offering | Proposed
maximum price(2) | Amount
of fee(2) | ||||||||||||
| Ordinary Shares, no par value | $ | $ | $ | |||||||||||||
| (1) | Pursuant to Rule 416 under the Securities Act of 1933, as amended, or the Securities Act, the ordinary shares registered hereby also include an indeterminate number of additional ordinary shares as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations or other similar transactions. |
| (2) | Estimated solely for purposes of calculating the amount of the registration fee pursuant to Rule 457(o) under the Securities Act, based upon the average of the high and low sales prices of the registrant’s Ordinary Shares as reported on the Tel Aviv Stock Exchange, or TASE, on , 2021. Share prices in New Israeli Shekels are translated (solely for the purpose of calculating the registration fee) using the rate of NIS to $1.00, the representative rate of exchange as of , 2021 as published by the Bank of Israel. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED JULY 6, 2021 |
91,885,555 Ordinary Shares

IceCure Medical Ltd.
This prospectus relates to the resale, by the selling shareholders identified in this prospectus of up to 91,885,555 ordinary shares, no par value, or Ordinary Shares, as further described below under “Prospectus Summary—Recent Private Placement.”
The selling shareholders are identified in the table commencing on page 111. No Ordinary Shares are being registered hereunder for sale by us. We will not receive any proceeds from the sale of the Ordinary Shares by the selling shareholders. All net proceeds from the sale of the Ordinary Shares covered by this prospectus will go to the selling shareholders (see “Use of Proceeds”). The selling shareholder are offering their securities in order to create a public trading market for our equity securities in the United States. Unlike an initial public offering, any sale by the selling shareholders of the Ordinary Shares is not being underwritten by any investment bank. The selling shareholders may sell all or a portion of the Ordinary Shares from time to time in market transactions through any market on which our Ordinary Shares are then traded, in negotiated transactions or otherwise, and at prices and on terms that will be determined by the then prevailing market price or at negotiated prices directly or through a broker or brokers, who may act as agent or as principal or by a combination of such methods of sale (see “Plan of Distribution”).
We have applied to list the Ordinary Shares on the Nasdaq Capital Market, or Nasdaq, under the symbol “ICCM.” This offering is contingent upon the listing of the Ordinary Shares on Nasdaq. Although we believe that as of the closing of the January 2021 SPA we meet the initial listing criteria for listing our Ordinary Shares on Nasdaq, no assurance can be given that our application will be approved or that a trading market in the United States will develop.
Our Ordinary Shares currently trade on the Tel Aviv Stock Exchange, or TASE, under the symbol “ICCM.” The last reported sale price of our Ordinary Shares on , 2021 was NIS , or approximately $ per share (based on the exchange rate reported by the Bank of Israel on such date).
We expect the opening price of our Ordinary Shares on Nasdaq to be determined based on the closing price of our Ordinary Shares on the TASE on , 2021, converted to U.S. dollars (based on the exchange rate reported by the Bank of Israel on such date).
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and a “foreign private issuer”, as defined in Rule 405 under the U.S. Securities Act of 1933, as amended, or the Securities Act, and are eligible for reduced public company reporting requirements.
Investing in our securities involves a high degree of risk. (see “Risk Factors” beginning on page 11).
Neither the Securities and Exchange Commission, or the SEC, the Israel Securities Authority nor any state or other foreign securities commission has approved nor disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2021
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. Neither we nor the selling shareholder have authorized anyone to provide you with different information. Neither we nor the selling shareholders are making an offer of these securities in any jurisdiction where the offer is not permitted. You should not assume that the information in this prospectus or any applicable prospectus supplement is accurate as of any date other than the date of the applicable document. Since the date of this prospectus and the documents incorporated by reference into this prospectus, our business, financial condition, results of operations and prospects may have changed.
For investors outside of the United States: Neither we nor any of the selling shareholders have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
In this prospectus, “we,” “us,” “our,” the “Company” and “IceCure” refer to IceCure Medical Ltd. and its wholly owned subsidiaries, IceCure Medical Inc., a Delaware corporation, IceCure Medical HK Limited a Hong Kong corporation and IceCure (Shanghai) MedTech Co., Ltd., a subsidiary of IceCure Medical HK Limited.
Our reporting currency and functional currency is the U.S. dollar. Unless otherwise expressly stated or the context otherwise requires, references in this prospectus to “NIS” are to New Israeli Shekels, and references to “dollars” or “$” mean U.S. dollars.
This prospectus includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we believe that these sources are reliable, we have not independently verified the information contained in such publications.
We report our financial statements in accordance with generally accepted accounting principles in the United States, or U.S. GAAP.
i
This prospectus describes the general manner in which the selling shareholders identified in this prospectus may offer from time to time up to 91,885,555 Ordinary Shares. If necessary, the specific manner in which the Ordinary Shares may be offered and sold will be described in a supplement to this prospectus, which supplement may also add, update or change any of the information contained in this prospectus. To the extent there is a conflict between the information contained in this prospectus and the prospectus supplement, you should rely on the information in the prospectus supplement, provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, any prospectus supplement—the statement in the document having the later date modifies or supersedes the earlier statement.
ii
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our securities. Before you decide to invest in our securities, you should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and related notes appearing at the end of this prospectus.
Our Company
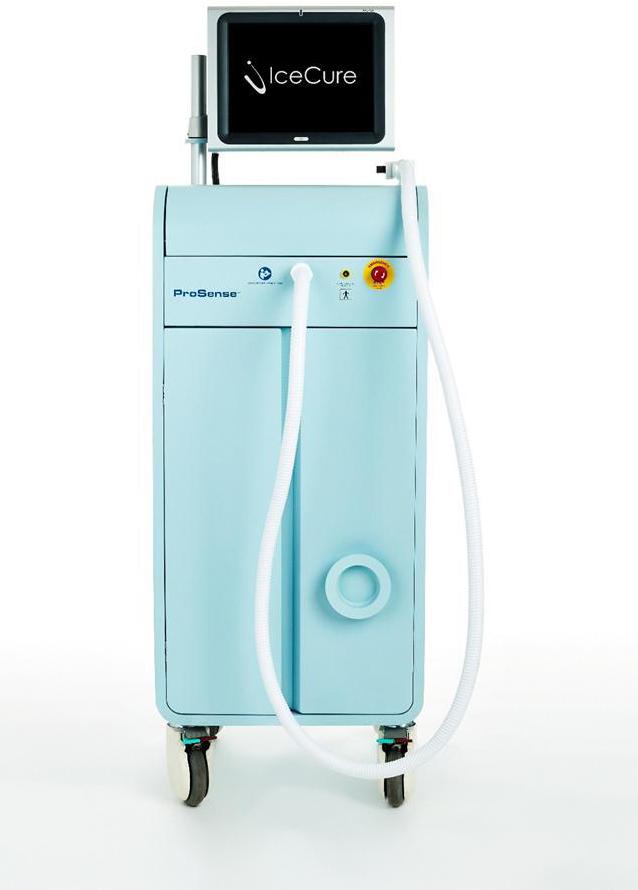
We are a commercial stage medical device company focusing on the research, development and marketing of cryoablation systems and technologies based on liquid nitrogen, or LN2, for treating tumors. Cryoablation is the process by which benign and malignant tumors are ablated (destroyed) through freezing such tumors while in a patient’s body. Our proprietary cryoablation technology is a minimally invasive alternative to surgical intervention, for tumors, including those found in breast, lungs, kidneys, bones and other indications. Our lead commercial cryoablation product is the ProSense system (pictured below).
In addition to our existing lead product, the ProSense system, a single probe system, we have developed an additional multi probe system that is expected to have the ability to freeze several tumors simultaneously or larger tumors, which we refer to as our MultiSense system. In our continued efforts aimed at improving our core technology, we are currently focusing on developing our next generation MultiSense system, which we intend to commercialize subject to regulatory approvals. We are also currently in the process of developing our next generation single probe system. While these next generation systems are still in various research and development stages, we expect them to be more efficient and user friendly (see “Business – Our Products – Research and Development” for additional information).
We believe that obtaining regulatory approval for our products for specific indications will help us grow our business. As of July 2021, we have received a broad range of regulatory approvals for our systems to treat tumors in the lungs, kidneys, bones and other indications. In the United States our products are approved as a “single family” known as the “IceCure Family,” which includes the IceSense3, ProSense, and MultiSense (which has not been commercialized) cryoablation systems. Although our existing, “IceCure Family” systems have regulatory approvals from the United States Food and Drug Administration, or the FDA, for commercialization in the United States, we have yet to receive regulatory approval for such systems for treatment of malignant breast tumors, which requires a separate approval from the FDA. The FDA classifies medical devices into one of three classes (Class I, Class II, or Class III) depending on their level of risk and the types of controls that are necessary to ensure device safety and effectiveness. The class assignment is a factor in determining the type of premarketing submission or application, if any, that will be required before marketing products in the United States. If the FDA does not approve 510(k) regulatory pathway, we will request that De Novo classification be accepted for our “IceCure Family” systems. If De Novo classification is needed for our “IceCure Family” systems, we will be required to accept special controls imposed by the FDA, mainly on the production process and post-market monitoring. If a De Novo classification is not approved by the FDA as the regulatory pathway, the FDA will accept only pre-market approval, or PMA, in which case we expect the timeline for marketing approvals would be longer and our costs associated with PMA would be higher compared to 510(k) or De Novo approvals (see “Business – Government Regulation” for additional information).
1
Obtaining regulatory approvals and developing our next generation MultSense system will require the incurrence of significant costs and our success will depend, in part, on gaining market acceptance. In order to gain market acceptance in the United States, we will require specific approval from the FDA for our ProSense system, which we hope to obtain based on the interim results of our ICE3 trial published on April 29, 2021 obtaining Medicare coverage from the Medicare Coverage of Innovative Technology, or MCIT, the American Society of Breast Surgeons, or the ASBrS, amending their guidelines to support cryoablation as an alternative to surgery, applying for CPT1 codes for cryoablation for breast cancer (with the support of the ASBrS), negotiate medical coverage for our systems from medical insurers and collaborating with a major distributor of medical devices (see “Business – Government Regulation – FDA Regulation of Medical Devices” and “Risk Factors – Risks Related to Product Development and Regulatory Approval” for additional information).
In addition, competition in the medical devices and cancer treatment market is intense. Some of our competitors hold significant market share, have long histories and strong reputations within the industry, greater brand recognition, financial and human resources than we do. They also have more experience and capabilities in researching and developing testing devices, obtaining and maintaining regulatory clearances and other requirements, manufacturing and marketing those products than we do. Their dominant market position and significant control over the market could significantly limit our ability to introduce or effectively market and generate sales of our ProSense and MultiSense systems.
To date, we have incurred significant operating losses, generated minimal revenues from product sales, and as of December 31, 2020, our accumulated deficit was $48.5 million. We expect that we will need to raise substantial additional funding in the future (see “Risk Factors – Risks Related to Our Financial Condition and Capital Requirements”).
In Europe, Singapore, India, Hong Kong, Russia, Thailand, Taiwan, South Africa, Mexico, Australia, Israel, Columbia and Costa Rica we have approvals for either our ProSense or our IceSense3 systems, or, in certain countries, both products. For instance, in China, we have approval for our IceSense3 (without the disposables) (see “Business – Our Products – Regulatory Approvals” below for additional information). In addition, in order to generate significant revenue, we are seeking to pursue additional regulatory approvals for our system for specific indications, in countries where we already have general regulatory approvals. In these countries, we are seeking regulatory approval for the treatment of specific tumors, including those found in breast, lungs, kidneys and bones. In addition, we are also seeking regulatory approvals for our system in other countries where we believe that there is significant potential for sales of our systems.
The procedure using our ProSense system begins with the introduction of our proprietary disposable probe into the tumor through a small pea-sized incision in the skin while the patient is under local anesthesia and/or sedation. The probe is guided by high-resolution ultrasound (for breast tumors) or computed tomography, or CT (for other indications). For some indications we use guiding needles (introducers) in order to guide the probe to the tumor. Once the probe is in place, LN2 is introduced into the probe in a closed-loop circuit, so that LN2 does not enter the body, and creates a freezing zone around the tip of the probe. During the freeze cycle, an ice ball forms in the freezing zone and encompasses the tumor, ablating the cancer or benign tumor, while the ice ball can be monitored by the physician using ultrasound or CT in order to avoid causing damage to the healthy tissue surrounding the tumor. Several minutes after the procedure is completed, the ice ball thaws and as a result thereof, there is no need for surgical removal of the dead tumor tissue, as the dead tissue is absorbed by the body in a natural process. The entire cryoablation procedure of freeze-thaw-freeze with our ProSense system generally takes between 15 to 40 minutes, depending on the size, type and location of the tumor. The same system configuration can be used and was designed to treat both malignant and non-malignant tumors. However, there is usually a different configuration for the probe handle between the systems used to treat breast tumors (with a primarily straight handle) and used to treat other indications (with a 90-degrees handle) due to the different imaging device that is used in each instance (see “Business – Our Products” for additional information). Pictured below is our system forming an ice ball (which in practice forms around the tissue within the body) in ultrasound gel.

2
As a minimally invasive alternative to surgery, cryoablation is much less traumatic than open surgery, and, based on current data, we believe this treatment is more affordable, entails less risk and generally results in fewer side effects and complications than open surgery. On the patient side, following procedures with our cryoablation technology, patients usually can resume normal activities within 24 hours after the procedure. In addition, in breast procedures with our technology, there is no need for a post-procedure reconstruction surgery. On the health provider side, procedures with our technology for breast tumors may be carried out in a clinic, while treatment by our ProSense system for other indications can generally be carried out as an outpatient procedure in a CT room. As a result, the potential profit margins for health care providers and payors are potentially greater than most of the current surgical procedures that are conducted in the operating room, which entail additional and expensive cost elements for the operating room and operating room staff. In addition, we believe that our LN2-based technology provides patients, medical service providers, physicians and insurers with advantages versus our competitors, especially those using heat to treat tumors, also known as thermal ablation (otherwise known as heat ablation). For example, the freezing effect on tissue from cryoablation produces less pain, and accordingly less anesthesia (which also reduces costs). A study published on April 8, 2021 by Elles M.F. van de Voort et al titled "Thermal Ablation as an Alternative for Surgical Resection of Small ( ≤2 cm) Breast Cancers: A Meta-Analysis" suggested that cryoablation has the lowest complication rate among breast cancer tumor procedures and the advantage of an analgesic effect. In addition, in comparison to the treatment of tumors with heat, when treating a tumor with cryoablation (unlike treatment with heat technology), the freezing does not cause evaporation of the treated tissue and therefore provides the physician conducting the treatment with a clearer view of the tissue, which we believe enables the physician to carry out the procedure more accurately with a precise view of the tumor.
We believe that cryoablation has already started to be recognized for its true potential, and that it represents the future of treatment for certain benign and malignant tumors. Our ongoing business strategy, as further detailed below, is focused on helping us overcome certain factors that have limited our ability to generate significant revenues, including, but not limited to, obtaining support of key opinion leaders and leading medical associations for the use of cryoablation (and specifically, our systems) for the treatment of tumors. In recent years, and in part due to our efforts and accomplishments, cryoablation of malignant breast tumors has been recognized for its vast potential. For example, following preliminary results of our ICE3 trial, in October 2018, the American Society of Breast Surgeons, or the ASBrS, which, among other things, sets guidelines for breast cancer treatment, updated its guidelines on performing cryoablation procedures on breast malignant tumors in their early stages. While not cited by the ASBrS, we believe that the results of our study were a factor in the ASBrS decision to update these guidelines. (see “Business – Our Lead Indication and Market Opportunity – Primary Indication – Breast Tumors – Malignant Breast Tumors – Multi-Site Clinical Trial of the Cryoablation System ICE3 Study – United States” for additional information).
In addition to updating its guidelines, the ASBrS also recommend taking part in clinical trials and in registries for treating malignant breast tumors, each relating to cryoablation, to increase the knowledge and data of breast cancer cryoablation. Registries, by which uniform data is collected from patients through observational studies, represent an important stage in the commercialization of technologies and are part of the required procedure for receiving remuneration from health insurance companies.
Recent Developments
COVID-19 Pandemic
In December 2019, a novel strain of coronavirus, or COVID-19, was identified in Wuhan, China. The spread of COVID-19 from China to over 200 countries, including the United States and Israel, resulted in the World Health Organization declaring the outbreak of COVID-19 as a “pandemic,” or a worldwide spread of a new disease.
Due to the burden on health systems and the fear of infection, medical institutions in certain territories restricted elective procedures, including those related to our area of activity. As a result, certain of our plans for 2020 and our plans for 2021 were impacted, including, for example, our ability to hold face to face meetings, conventions and on-site trainings. In addition, although we were able to have certain face to face meetings and hold virtual meetings, our ability to reach new customers was impacted due to travel restrictions and social distancing requirements. We believe that due to the COVID-19 pandemic, investments in new technologies, including, for example, our ProSense system, decreased as customers sought to minimize their capital expenditures. Despite the resulting effects of the COVID-19 pandemic, we do not believe that we have sustained any material adverse effect on our financial condition and operations, and in spite of the COVID-19 pandemic, our revenues for our fiscal year ended December 31, 2020 increased by 138% in comparison to the year ended December 31, 2019 (see “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” for additional information). We continued to face challenges during the first quarter of 2021, and expect to continue to experience COVID-related challenges for the foreseeable future, mainly related to delays in shipments of materials by our suppliers, and disruption in access to new medical service providers and costumers due to the continued restrictions on international travel, direct access to medical service providers and public gatherings. Any disruption of suppliers or access to medical service provider would likely impact our progress as well as our ability to access capital through the financial markets.
3
Although we were not able to increase sales to new customers as planned prior to the onset of the COVID-19 pandemic, we believe that certain competitive advantages that our ProSense system has versus surgical treatment helped us generate sales to our existing customers, and avert any material adverse effect on our financial condition or operations.
We continue to examine the consequences of the COVID-19 pandemic, perform risk assessments and implement operational solutions that we believe will help us deal with the COVID-19 pandemic, we are unable to accurately predict the impact that the COVID-19 pandemic will have on our operations going forward due to uncertainties that will be dictated by the length of time that the COVID-19 pandemic and related disruptions continue, the impact of governmental regulations that might be imposed in response to such pandemic and overall changes in the behavior of our customers.
Recent Private Placement
Pursuant to a securities purchase agreement, or the January 2021 SPA, with certain investors, or the January 2021 Investors, we received an aggregate amount of $9 million, against the issuance of 55,131,333 Ordinary Shares, or the January 2021 First Closing.
Pursuant to the January 2021 SPA, a second closing, or the January 2021 Second Closing, in the aggregate amount of $6 million, against issuance of 36,754,222 Ordinary Shares was to occur upon the approval of our Ordinary Shares for listing on a tier of the Nasdaq Stock Market and the effectiveness of a registration statement covering the resale of the Ordinary Shares, or the Nasdaq Milestone, which we undertook to file with the SEC within 120 days from the January 2021 First Closing. However, on May 9, 2021, the January 2021 Investors waived the achievement of the Nasdaq Milestone as a pre-condition, and we completed the January 2021 Second Closing.
The January 2021 Investors were granted a 12-month participation right following the January 2021 Second Closing, in future financings equal to 50% of the subsequent financing, subject to certain conditions. We also undertook to refrain from issuing any Ordinary Shares or Ordinary Shares equivalents from the date of the January 2021 SPA until 60 calendar days from the January 2021 Second Closing, subject to certain exempt issuances.
Summary Risk Factors
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary. You should read these risks in full before you invest in our securities. The following is a summary of such risks.
Risks Related to Our Financial Condition and Capital Requirements
| ● | we have a limited operating history and we have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future; | |
| ● | we have generated minimal revenues from product sales and may never be profitable, even if we receive regulatory approval to commercialize our products in additional geographical territories and indications; | |
| ● | even following the January 2021 SPA, we expect that we will need to raise substantial additional funding, which may not be available on acceptable terms, or at all. Failure to obtain funding on acceptable terms and on a timely basis may require us to curtail, delay or discontinue our commercialization and product development efforts, expansion to new markets, or other activities. |
4
Risks Related to Our Business and Industry
| ● | we are highly dependent on the successful development, marketing and sale of our ProSense and MultiSense systems; | |
| ● | we face business disruption and related risks resulting from the recent outbreak of the COVID-19 pandemic, which could have a material adverse effect on our business and results of operations; | |
| ● | if we fail to maintain existing strategic relationships with Terumo Corporation or are unable to identify additional strategic distributors of our systems, and any future products and technologies, our revenues may decrease; | |
| ● | we are dependent upon third-party manufacturers and suppliers making us vulnerable to supply shortages and problems, increased costs and quality or compliance issues, any of which could harm our business; | |
| ● | if we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks; | |
| ● | we manage our business through a small number of employees and key consultants. We may need to expand our organization and we may experience difficulties in recruiting needed additional employees and consultants, which could disrupt our operations; | |
| ● | we face intense competition in the market, and as a result we may be unable to effectively compete in our industry; | |
| ● | our commercial success is very much dependent on third-party payors to provide adequate insurance coverage and reimbursement for the use of our systems, or any future products that we may commercialize; | |
| ● | our management team has limited experience managing a U.S. reporting company; |
Risks Related to Product Development and Regulatory Approval
| ● | our, or our partners’, clinical trials may encounter delays, suspensions or other problems; | |
| ● | we may not receive, or may be delayed in receiving, the necessary clearances or approvals for our current products or future products in order to commercialize these products in specific countries or regions or in a specific indication, and failure to timely obtain necessary clearances or approvals for our existing or future products would adversely affect our ability to grow our business; | |
| ● | preliminary data that we or others announce or publish from time to time with respect to our products may change as more data become available and are subject to audit and verification procedures that could result in material changes in the final data; | |
| ● | the misuse or off-label use of our products may harm our reputation in the marketplace, result in injuries that may lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly; | |
| ● | our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to immediately report to all relevant regulatory authorities, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us; | |
| ● | if we do not obtain and maintain international regulatory registrations, clearances or approvals for our products, we will be unable to market and sell our products; | |
| ● | disruptions at the FDA and other government agencies could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being cleared or approved or commercialized in a timely manner or at all, which could negatively impact our business; |
5
Risks Related to Our Intellectual Property
| ● | if we are unable to obtain and maintain effective patent rights for our products and services, we may not be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us; | |
| ● | third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts; |
Risks Related to the Ownership of our Ordinary Shares
| ● | our principal shareholders, officers and directors currently beneficially own approximately 75.81% of our Ordinary Shares. They will therefore be able to exert significant control over matters submitted to our shareholders for approval; | |
| ● | we may be a “passive foreign investment company,” or PFIC, for U.S. federal income tax purposes in the current taxable year or may become one in any subsequent taxable year. There generally would be negative tax consequences for U.S. taxpayers that are holders of the Ordinary Shares if we are or were to become a PFIC; |
Risks Related Israeli Law and Our Operations in Israel
| ● | potential political, economic and military instability in the State of Israel, where our headquarters, members of our management team and our research and development facilities are located, may adversely affect our results of operations; | |
| ● | We expect to be exposed to fluctuations in currency exchange rates, which could adversely affect our results of operations; | |
| ● | the termination or reduction of tax and other incentives that the Israeli government provides to Israeli companies may increase our costs and taxes; | |
| ● | we may be required to pay monetary remuneration to our Israeli employees for their inventions, even if the rights to such inventions have been duly assigned to us. We may also not be able to enforce covenants not-to-compete under current Israeli law that might result in added competition for our products; | |
| ● | we received Israeli government grants for certain of our research and development activities, the terms of which may require us to pay royalties and to satisfy specified conditions in order to manufacture products and transfer technologies outside of Israel. If we fail to satisfy these conditions, we may be required to pay penalties and refund grants previously received; | |
| ● | provisions of Israeli law and our amended and restated articles of association may delay, prevent or otherwise impede a merger with, or an acquisition of, us, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders; | |
| ● | it may be difficult to enforce a judgment of a U.S. court against us, our executive officers and directors and the Israeli experts named in this prospectus in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our executive officers and directors and these experts; and | |
| ● | your rights and responsibilities as a shareholder will be governed in key respects by Israeli laws, which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies |
6
Corporate Information
We are an Israeli corporation based in Caesarea, Israel and were incorporated in Israel in 2006. On February 2, 2011, we became a public company in Israel and our shares were listed for trade on the TASE. Our principal executive offices are located at 7 Ha’Eshel St., PO Box 3163, Caesarea, 3079504 Israel. Our telephone number in Israel is +972-4-6230333. Our website address is http://www.icecure-medical.com. The information contained on, or that can be accessed through, our website is not part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
This prospectus contains trademarks, trade names and service marks, which are the property of their respective owners. Solely for convenience, trademarks, trade names and service marks referred to in this prospectus may appear without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent permitted under applicable law, our rights or the right of the applicable licensor to these trademarks, trade names and service marks. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the JOBS Act. As such, we are eligible to, and intend to, take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not “emerging growth companies” such as not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. We could remain an “emerging growth company” for up to five years, or until the earliest of (a) the last day of the first fiscal year in which our annual gross revenues exceeds $1.07 billion, (b) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, which would occur if the market value of our Ordinary Shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (c) the date on which we have issued more than $1 billion in nonconvertible debt during the preceding three-year period.
Implications of being a “Foreign Private Issuer”
We are subject to the information reporting requirements of the Exchange Act that are applicable to “foreign private issuers,” and under those requirements we file reports with the SEC. As a foreign private issuer, we are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. For example, we are not required to issue quarterly reports, proxy statements that comply with the requirements applicable to U.S. domestic reporting companies, or individual executive compensation information that is as detailed as that required of U.S. domestic reporting companies. We also have four months after the end of each fiscal year to file our annual report with the SEC and are not required to file current reports as frequently or promptly as U.S. domestic reporting companies. Our officers, directors and principal shareholders are exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. As a foreign private issuer, we are not subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act. In addition, as a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the Nasdaq Stock Market rules for domestic U.S. issuers and are not required to be compliant with all Nasdaq Stock Market rules as of the date of our initial listing on Nasdaq as would domestic U.S. issuers. (see “Risk Factors—Risks Related to the Ownership of Our Securities”). These exemptions and leniencies will reduce the frequency and scope of information and protections available to you in comparison to those applicable to a U.S. domestic reporting company. We intend to take advantage of the exemptions available to us as a foreign private issuer during and after the period we qualify as an “emerging growth company.”
7
THE OFFERING
This prospectus relates to the resale by the selling shareholders identified in this prospectus of up to 91,885,555 Ordinary Shares. All of the Ordinary Shares, when sold, will be sold by these selling shareholders. The selling shareholders may sell their Ordinary Shares from time to time at prevailing market prices. We will not receive any proceeds from the sale of the Ordinary Shares by the selling shareholders.
| Ordinary Shares currently issued and outstanding | 254,181,695 Ordinary Shares |
Ordinary Shares offered by the selling Shareholders |
Up to 91,885,555 Ordinary Shares. |
| Use of proceeds | We will not receive any proceeds from the sale of the Ordinary Shares by the selling shareholders. All net proceeds from the sale of the Ordinary Shares covered by this prospectus will go to the selling shareholders (see “Use of Proceeds”). |
| Risk factors | You should read the “Risk Factors” section starting on page 11 of this prospectus for a discussion of factors to consider carefully before deciding to invest in our securities. |
| TASE symbol | “ICCM” | |
| Proposed Nasdaq Capital Market symbol | We have applied to list our Ordinary Shares on Nasdaq under the symbol “ICCM.” Although we believe that as of the closing of the January 2021 SPA we met the initial listing criteria for listing our Ordinary Shares on Nasdaq, there can be no assurance that our application will be approved. |
The number of the Ordinary Shares to be outstanding immediately after this offering excludes:
| ● | 11,785,912 Ordinary Shares issuable upon the exercise of options to directors, employees and consultants under our share incentive plan, outstanding as of July 6, 2021, at a weighted average exercise price of $0.21, of which 6,608,049 were vested as of July 6, 2021; and | |
| ● | 17,100 Ordinary Shares issuable upon the exercise of warrants to directors, employees and consultants, outstanding as of July 6, 2021, at a weighted average exercise price of $0.31. |
8
SUMMARY CONSOLDIATED FINANCIAL DATA
The following table summarizes our consolidated financial data. We have derived the following statements of operations data for the years ended December 31, 2020 and 2019 and balance sheet data as of December 31, 2020 and 2019 from our audited financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. The following summary financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| Year
Ended December 31, |
||||||||
| U.S. dollars in thousands, except share and per share data | 2020 | 2019 | ||||||
| Revenues | 3,868 | 1,627 | ||||||
| Cost of revenues | 1,424 | 1,103 | ||||||
| Gross profit | 2,444 | 524 | ||||||
| Research and development expenses | 3,809 | 3,001 | ||||||
| Sales and marketing expenses | 1,063 | 1,035 | ||||||
| General and administrative expenses | 1,714 | 1,272 | ||||||
| Operating loss | 4,142 | 4,784 | ||||||
| Financial income, net | (412) | (233 | ) | |||||
| Net loss and comprehensive loss | 3,730 | 4,551 | ||||||
| Basic and diluted net loss per Ordinary Share | 0.027 | 0.042 | ||||||
| Weighted average number of shares outstanding used in computing basic and diluted loss per share | 137,031,221 | 108,970,277 | ||||||
9
| As
of December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Balance Sheet Data: | ||||||||
| Cash and cash equivalents | $ | 3,502 | $ | 5,789 | ||||
| Deposit | 4,669 | - | ||||||
| Trade accounts receivable | 94 | 17 | ||||||
| Inventory | 1,064 | 678 | ||||||
| Prepaid expenses and other receivables | 260 | 381 | ||||||
| Total current assets | 9,589 | 6,865 | ||||||
| NON-CURRENT ASSETS | ||||||||
| Prepaid expenses and other long-term assets | 37 | 39 | ||||||
| Right of use assets | 306 | 225 | ||||||
| Property and equipment, net | 307 | 147 | ||||||
| Total non-current assets | 650 | 411 | ||||||
| Total assets | 10,239 | 7,276 | ||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Trade accounts payable | 645 | 428 | ||||||
| Lease liabilities | 214 | 135 | ||||||
| Other current liabilities | 2,855 | 2,990 | ||||||
| Total current liabilities | 3,714 | 3,553 | ||||||
| NON-CURRENT LIABILITIES | ||||||||
| Long term lease liability | 118 | 105 | ||||||
| Other long-term liabilities | 759 | 327 | ||||||
| Total non-current liabilities | 877 | 432 | ||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Ordinary Shares, No par value; Authorized 20,000,000,000 shares; Issued and outstanding: 161,745,754 shares 120,235,754 shares as of December 31, 2020 and December 31, 2019, respectively. | - | 3,318 | ||||||
| Treasury shares | (41) | (41 | ) | |||||
| Additional paid-in capital | 54,225 | 44,820 | ||||||
| Accumulated deficit | (48,536) | (44,806 | ) | |||||
| Total shareholders’ equity | 5,648 | 3,291 | ||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | 10,239 | 7,276 | ||||||
10
Investing in our securities involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including the financial statements and related notes, before deciding whether to purchase our securities. If any of the following risks are realized, our business, operating results, financial condition and prospects could be materially and adversely affected. In that event, the price of our Ordinary Shares could decline, and you could lose part or all of your investment.
Risks Related to Our Financial Condition and Capital Requirements
We have a limited operating history and we have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future. The report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern, which could prevent us from obtaining new financing on reasonable terms or at all.
We are a medical device company with a limited operating history. To date, we have focused on developing our first commercial product for cryoablating tumors, the ProSense system, collecting clinical data, obtaining regulatory approvals in different geographical territories and indications and initiated our commercialization effort. We have funded our operations to date primarily through raising capital on TASE, private offerings, minimal sales of our ProSense system and its components, including affiliated needles, or Probes, guiding needles, or Introducers and other products, which we collectively refer to as disposables, loans, convertible loans and royalty-bearing grants that we received from the Israeli Innovation Authority, or the IIA, formerly known as the Office of the Chief Scientist of the Ministry of Economy and Industry.
We have only a limited operating history upon which you can evaluate our business and prospects. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the medical device industry. To date, we have generated minimal revenues from the sale of our ProSense system and its components (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” for additional information). We have incurred losses in each year since our inception, including operating losses of $4,142 thousand and $4,784 thousand for the years ended December 31, 2020 and 2019, respectively. As of December 31, 2020, we had an accumulated deficit of $48,536 thousand. Substantially all of our operating losses resulted from costs incurred in connection with our development of our technology, business development and commercialization and from general and administrative costs associated with our operations.
Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through debt or equity financing. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to our products.
We expect our research and development expenses to increase in connection with our planned expanded research and development efforts, including those conducted in connection with the development of our next generation single Probe and MultiSense systems, and as we seek to receive approval from applicable regulatory authorities to commence commercialization of our ProSense system for treatment in breast cancer and other indications. In addition, if we obtain marketing approval for our ProSense system for the treatment of breast cancer and other indications in different countries across the world, including the United States, and for our next generation single Probe and MultiSense systems, we will likely incur significant sales, marketing and outsourced manufacturing expenses. In addition, although we have certain regulatory approvals, these only allow us to conduct minimal commercialization of our products, and therefore we will need to seek additional regulatory approvals in order to initiate commercialization, in scale, that has the potential to generate significant revenues for us. Even if we were to receive marketing approval for our ProSense and MultiSense systems, we expect that we will continue to incur significant research and development expenses as we seek to improve our technology and effectively compete with our competitors and as we seek additional approvals for their use in different indications and marketing and commercialization costs.
Furthermore, in addition to such operating expenses, we expect to incur additional costs associated with the process of listing our Ordinary Shares on Nasdaq and operating as a public company subject to the rules and regulations of the SEC, which we estimate will be at least one million dollars annually. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing a medical device, we are unable to predict the extent of any future losses or when we will become profitable, if at all.
11
The regulatory marketing approvals that we currently have are insufficient to generate significant revenue. Therefore, we expect to continue to incur significant losses until we are able to meaningfully commercialize our ProSense system or our next generation single Probe and MultiSense systems, which we may not be successful in achieving. We anticipate that our expenses will increase substantially if and as we:
| ● | continue the research and development of our technology; | |
| ● | discover that there are robust technology changes in our field; | |
| ● | seek regulatory and marketing approvals for our medical devices, and more specifically, our ProSense system for treatment of breast cancer; | |
| ● | subject to the receipt of the applicable regulatory approvals, establish and expand a sales, marketing, and distribution infrastructure to commercialize our current ProSense system and our future next generation single Probe and MultiSense systems, and its disposables; | |
| ● | seek to identify, assess, acquire, license, and/or develop other medical devices companies and subsequent generations of our current medical devices; | |
| ● | seek to maintain, protect, and expand our intellectual property portfolio; | |
| ● | seek to attract and retain skilled personnel; | |
| ● | create additional infrastructure to support our operations as a public company and our product development and planned future commercialization efforts; and | |
| ● | experience any delays or encounter issues with respect to any of the above, including, but not limited to, failed studies, complex results, safety issues or other regulatory challenges that require longer follow-up of existing studies or additional supportive studies in order to pursue marketing approval. |
The amount of any future operating losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through sales, equity or debt financings, strategic collaborations or grants. Even if we obtain regulatory approval to market our ProSense system or any future products, including the next generation single Probe and MultiSense systems, our future revenues will depend on the market size (geographic and indication-specific) in which any such product receives approval and our ability to achieve sufficient market acceptance, competition, pricing, reimbursement from third-party payors for our ProSense and next generation single Probe and MultiSense systems or any future product candidates. Further, the operating losses that we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. Other unanticipated costs may also arise.
We have generated minimal revenues from product sales and may never be profitable, even if we receive regulatory approval to commercialize our products in additional geographical territories and indications.
Our system and its disposables are approved for marketing in a limited number of jurisdictions and for use in treatment of certain indications. In order to generate significant revenue, we will need to obtain additional regulatory approvals in jurisdictions within which we already have certain regulatory approvals and also in jurisdictions in which we currently have no regulatory approvals to market our products. Even if our ProSense or MultiSense systems or any future products are approved for marketing and sale, we anticipate incurring significant incremental costs associated with commercializing such products.
Our ProSense system, and its disposables, has regulatory approvals that allow us to market our system or its disposables in certain geographical areas and for specific indications. However, even with these regulatory approvals in place, we have yet to generate significant revenues and we plan to seek for additional regulatory approvals covering additional clinical indications, to allow us to increase clinical acceptance of our products by the medical community, obtain reimbursement coverage, and partner with distributors, all in order to increase commercialization efforts (see “Business—Government Regulation” for additional information). However, there can be no assurance that we will obtain regulatory approvals for all indications we have applied, or intend to apply for, or at all.
12
In addition to our dependency on receiving adequate regulatory approvals to market our products to our target market (geographic and indication-specific), our ability to generate significant revenues and achieve profitability also depends on our success in many areas, including but not limited to:
| ● | complete research and development of our MultiSense and ProSense systems and any future products in a timely and successful manner; | |
| ● | obtain market acceptance, if and when approved, of our ProSense and MultiSense systems and any future products from the medical community, patients and third-party payors; | |
| ● | enter into agreements with commercial partners; | |
| ● | obtain sufficient clinical evidence from our trials and commercial procedures, and publish such data; | |
| ● | maintain and enhance a commercially viable, sustainable, scalable, reproducible and transferable manufacturing process for our ProSense and next generation single Probe and MultiSense systems and any future product candidates that is compliant with current good manufacturing practices, or cGMPs, or any other applicable regulations or standards; | |
| ● | establish and maintain supply and, if applicable, manufacturing relationships with third parties that can provide, in both amount and quality, adequate products to support development and the market demand for our ProSense and next generation single Probe and MultiSense systems and any future products, if and when approved for marketing by regulators; | |
| ● | maintain sufficient average selling price for our products and the revenues margin that we generate; | |
| ● | launch and commercialize any products for which we obtain regulatory and marketing approval, either directly by establishing a sales force, marketing and distribution infrastructure, and/or with collaborators or distributors; | |
| ● | accurately identifying demand for our ProSense and next generation single Probe and MultiSense systems or any future products; | |
| ● | ensure our products are approved for reimbursement from governmental agencies, health care providers and insurers in jurisdictions where they have been approved for marketing; | |
| ● | address any competing technological and market developments that impact our technology or its prospective usage by medical professionals; | |
| ● | negotiate favorable terms in any collaboration, licensing or other arrangements into which we may enter and perform our obligations under such collaborations; | |
| ● | attract, hire and retain qualified personnel; and | |
| ● | locate and lease or acquire suitable facilities to support our clinical development, manufacturing facilities and commercial expansion. |
In addition, even if we were to receive all of the regulatory approvals that we may seek to receive, our expenses could increase beyond expectations if we are required by the FDA, or other regulatory agencies, domestic or foreign, to change our manufacturing processes or assays or to perform studies in addition to those that we currently anticipate.
13
Further, if we are not able to generate significant revenues from the sale of our approved products, we may be forced to curtail or cease our operations. Due to the numerous risks and uncertainties involved in product development, it is difficult to predict the timing or amount of increased expenses, or when, or if, we will be able to achieve or maintain profitability.
Even following the January 2021 SPA, we expect that we will need to raise substantial additional funding, which may not be available on acceptable terms, or at all. Failure to obtain funding on acceptable terms and on a timely basis may require us to curtail, delay or discontinue our commercialization and product development efforts, expansion to new markets, or other activities.
As of December 31, 2020, our cash and cash equivalents and deposits were approximately $8.17 million, and we had a working capital of $5,875 thousand and an accumulated deficit of $48,536 thousand. On March 9, 2021 and May 10, 2021 pursuant to the January 2021 SPA, we received $9,000 thousand, and $6,000 thousand, accordingly. We expect that our existing cash, cash equivalents and short-term deposits will be sufficient for the next 12 months of operation. We expect that we will require substantial additional capital to commercialize our ProSense system and to develop and commercialize our next generation single Probe and MultiSense systems. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future funding requirements will depend on many factors, including but not limited to:
| ● | the cost, timing and outcomes of regulatory review of ProSense system and any future products; | |
| ● | the costs of maintaining our own commercial-scale cGMP manufacturing facility, including costs related to obtaining and maintaining regulatory compliance, and/or engaging third-party manufacturers therefor; | |
| ● | the scope, progress, results and costs of product development, testing, manufacturing, preclinical development and, if applicable, clinical trials for any other products that we may develop or otherwise obtain in the future; | |
| ● | the cost of our future activities, including establishing sales, marketing and distribution capabilities for any products in any particular geography where we receive marketing approval for such products; | |
| ● | the terms and timing of any collaborative, licensing and other arrangements that we may establish; | |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; and | |
| ● | the level of revenues received from commercial sales of any product candidates for which we receive marketing approval. |
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our ProSense and next generation single Probe and MultiSense systems and any future product candidates. We cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all, during or after the COVID-19 pandemic. In addition, our ability to raise capital could be affected by various factors, including clinical adverse events. Moreover, the terms of any financing may adversely affect the holdings or the rights of holders of our securities and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our Ordinary Shares to decline. The incurrence of indebtedness could result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable, and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Even if we believe that we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the development or commercialization of our ProSense or next generation single Probe and MultiSense systems or any other products or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
14
Risks Related to Our Business and Industry
We are highly dependent on the successful development, marketing and sale of our ProSense and next generation single Probe and MultiSense systems.
Our ProSense system, our second generation cryoablation system, is the basis of our business. As a result, the success of our business plan is highly dependent on our ability to manufacture our ProSense at a large scale, and commercialize our ProSense system for the treatment of breast cancer, and other intended uses in the field of interventional oncology (including kidney cancer, lung cancer, liver cancer and bone cancer) and our failure to do so could cause our business to fail. Successful production and commercialization of medical devices is a complex and uncertain process, dependent on the efforts of management, manufacturers, local operators, integrators, medical professionals, third-party payors, as well as general economic conditions, among other factors. Any factor that adversely impacts the production and commercialization of our ProSense system, will have a negative impact on our business, financial condition, results of operations and prospects. We have limited experience in commercializing our ProSense system and we may face several challenges with respect to our commercialization efforts, including, among others, that:
| ● | we may not have adequate financial or other resources to complete the development of our next generation single Probe and MultiSense systems or any future products; | |
| ● | we may not be able to manufacture our ProSense system in commercial quantities, at an adequate quality or at an acceptable cost; | |
| ● | we may not be able to establish adequate sales, marketing and distribution channels for our products; | |
| ● | healthcare professionals medical providers and patients may not accept our products; | |
| ● | we may not be aware of possible complications from the continued use of our ProSense system since we have limited clinical experience with respect to the actual use of our ProSense system; | |
| ● | technological breakthroughs solutions in the ablation of tissues may reduce the demand for our ProSense system; | |
| ● | third-party payors may not agree to reimburse sufficiently, or at all patients or healthcare providers for any or all of the procedures conducted with our ProSense system, which may adversely affect medical providers, and patients’ willingness to use our ProSense system; | |
| ● | we may face third-party claims of intellectual property infringement; | |
| ● | we may fail to obtain or maintain regulatory clearance or approvals in our target markets (geographic and indication-specific) or may face adverse regulatory or legal actions even if regulatory approval is obtained; | |
| ● | prices may adversely affect patients’ willingness to use our ProSense system; and | |
| ● | guidelines published by the medical community may not recommend the use of our ProSense and next generation single Probe and MultiSense systems or any future products for certain indications, which may adversely affect healthcare users willingness to use our ProSense and next generation single Probe and MultiSense systems or any future products. |
If we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our products could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
15
We face business disruption and related risks resulting from the recent outbreak of the COVID-19 pandemic, which could have a material adverse effect on our business and results of operations.
The outbreak of COVID-19, which originated in Wuhan, China in 2019, has since spread across the globe, including the United States, Japan, Israel, many European and other countries in which we operate. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. While COVID-19 is still spreading and the final implications of the pandemic are difficult to estimate at this stage, it is clear that it has affected the lives of a large portion of the global population. At this time, the pandemic has caused states of emergency to be declared in various countries, travel restrictions imposed globally, quarantines established in certain jurisdictions and various institutions and companies being closed. We are actively monitoring the pandemic and we are taking any necessary measures to respond to the situation in cooperation with the various stakeholders.
COVID-19 infection of our workforce could result in a temporary disruption in our business activities, including manufacturing, and other functions. Based on guidelines provided by the Israeli government, employers (including us) are also required to prepare and increase as much as possible the capacity and arrangement for employees to work remotely. In that regard, while we continued to operate almost fully including carrying out our studies in compliance with all applicable Israeli rules and guidelines, our employees worked remotely when full lockdowns were enforced. In addition, the closure of borders and state mandated quarantines, have made it difficult for us to assist in the installation and maintenance of our ProSense and providing clinical hands-on support and were required to provide such services remotely.
The spread of an infectious disease, including COVID-19, may also result in the inability of our manufacturers to deliver components or finished products on a timely basis and may also result in the inability of our suppliers to deliver the parts required by our manufacturers to complete manufacturing of components or finished products. Since March 2020 we have experienced delays in supply of some components of our products from abroad. While we were able to overcome such delays, we might not be able to do so if the future. In addition, governments and medical service providers may divert spending from other budgeted resources as they seek to reduce and/or stop the spread of an infectious disease, such as COVID-19. Such events may result in a period of business and manufacturing disruption, and in reduced operations, any of which could materially affect our business, financial condition and results of operations. The extent to which COVID-19 impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others.
If we fail to maintain existing strategic relationships with Terumo Corporation or are unable to identify additional distributors of our products or any future products and technologies, our revenues may decrease.
We currently derive a significant amount of our revenues through our strategic relationships and exclusive distribution agreements with Terumo Corporation (including its affiliates). If our relationships with Terumo Corporation are terminated or impaired for any reason and we are unable to replace these relationships with other means of distribution, we could suffer a material decrease in revenues.
We may need, or decide it is otherwise advantageous to us, to obtain the assistance of additional distributors to market and distribute our future products and technologies, as well as to market and distribute our existing ProSense and next generation single Probe and MultiSense systems, to existing or new markets or geographical areas. We may not be able to find additional distributors who will agree to and are able to successfully market and distribute our systems and technologies on commercially reasonable terms, if at all. If we are unable to establish additional distribution relationships on favorable terms, our revenues may decline. In addition, our distributors may choose to favor the products of our competitors over ours and give preference to them.
Also, our financial results are dependent upon the service efforts of Terumo Corporation. If Terumo Corporation is unsuccessful in adequately servicing our products, our sales could significantly decrease and our business, financial condition, results of operations and prospects may be adversely impacted.
Pursuant to our agreements with Terumo Corporation, we are also dependent on Terumo Corporation’s efforts to obtain regulatory approval for the marketing and sale and the reimbursement of our products in Japan and other territories in which it seeks to commercialize our ProSense system, such as Thailand. If Terumo Corporation fails to obtain such approvals, it might adversely impact our future plans for sales in Japan and other regions.
16
Medical device development is costly and involves continual technological change which may render our current or future products obsolete.
The market for medical device technologies and products is characterized by factors such as rapid technological change, medical advances, changing consumer requirements, short device lifecycles, changing regulatory requirements and evolving industry standards. Any one of these factors could reduce the demand for our devices or require substantial resources and expenditures for, among other things, research, design and development, to avoid technological or market obsolescence.
Our success will depend on our ability to enhance our current technology and develop or acquire new technologies to keep pace with technological developments and evolving industry standards, while responding to changes in customer needs. A failure to adequately develop or acquire device enhancements or new devices that will address changing technologies and customer requirements adequately, or to introduce such devices on a timely basis, may have a material adverse effect on our business, financial condition and results of operations.
We might have insufficient financial resources to improve our ProSense system or complete the development of our next generation single Probe and MultiSense systems, and any other future products, and advance technologies and develop new devices at competitive prices. Technological advances by one or more competitors or future entrants into the field may result in our present services or devices becoming non-competitive or obsolete, which may decrease revenues and profits and adversely affect our business and results of operations.
We may encounter significant competition across our product lines and in each market in which we will sell our products and services from various companies, some of which may have greater financial and marketing resources than we do. Our competitors may include any companies engaged in the research, development, manufacture, and marketing of non-invasive or minimal invasive solutions and technologies to treat tumors, as well as a wide range of medical device companies that sell a single or limited number of competitive products and services or participate in only a specific market segment.
We will be dependent upon success in our customer acquisition strategy.
Our business will be dependent upon success in our customer acquisition strategy. If we fail to maintain a high quality of device technology, we may fail to retain or add new customers. If we fail, our revenue, financial results and business may be significantly harmed. Our future success depends upon expanding our commercial operations in the United States, Europe and South East Asia, as well as entering additional markets (geographic and indication-specific) to commercialize our next generation single Probe and MultiSense systems and any other future products. We believe that our expanded growth will depend on the further development, regulatory approval(s) and commercialization of our ProSense and next generation single Probe and MultiSense systems. If we fail to commercialize our products in a timely manner and across a range of indications, including breast cancer, we may not be able to expand our markets or to grow our revenue, and our business and financial condition may be adversely impacted. If medical practitioners do not perceive our products to be useful and reliable, we may not be able to attract or retain new customers. A decrease in sales growth could cause us to enter into sales or distribution agreements on terms less favorable to us or cause us to license our technology on unfavorable and unexpected terms, which may have a material and adverse impact on our revenue, business, reputation, financial condition and results of operations.
We are dependent upon third-party manufacturers and suppliers making us vulnerable to supply shortages and problems, increased costs and quality or compliance issues, any of which could harm our business.
We rely on third parties to manufacture and supply us with proprietary custom components. We rely on a limited number of suppliers who provide us materials and components as well as manufacture and assemble certain components of our products. Our suppliers may encounter problems during manufacturing for a variety of reasons, including, for example, failure to follow specific protocols and procedures, failure to comply with applicable legal and regulatory requirements, equipment malfunction and environmental factors, failure to properly conduct their own business affairs, infringement of third-party intellectual property rights, and as a result of the COVID-19 pandemic and the resulting government restrictions, any of which could delay or impede their ability to meet our requirements. Our reliance on these third-party suppliers also subjects us to other risks that could harm our business, including:
| ● | we are not currently a major customer of many of our suppliers, and these suppliers may therefore give other customers’ needs higher priority than ours; | |
| ● | third parties may threaten or enforce their intellectual property rights against our suppliers, which may cause disruptions or delays in shipment, or may force our suppliers to cease conducting business with us; |
17
| ● | we may not be able to obtain an adequate supply in a timely manner or on commercially reasonable terms; | |
| ● | our suppliers, especially new suppliers, may make errors in manufacturing that could negatively affect the efficacy or safety of our products or cause delays in shipment; | |
| ● | we may have difficulty locating and qualifying alternative suppliers; | |
| ● | switching components or suppliers may require product redesign, validation or verification processes and possibly submission to the FDA or other similar foreign regulatory agencies, which could significantly impede or delay our commercial activities; | |
| ● | one or more of our suppliers may be unwilling or unable to supply components of our products; | |
| ● | the occurrence of a fire, natural disaster or other catastrophe impacting one or more of our suppliers may affect their ability to deliver products to us in a timely manner; and | |
| ● | our suppliers may encounter financial or other business hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements. |
We consistently monitor our inventory levels and maintain recovery plans to address potential disruptions that we may encounter from our suppliers. However, we may not be able to quickly establish additional or alternative suppliers if necessary, in part because we may need to undertake additional activities to establish such suppliers as required by the regulatory approval process. Any interruption or delay in obtaining products from our third-party suppliers, or our inability to obtain products from qualified alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to switch to competing products. Given our reliance on certain suppliers, we may be susceptible to supply shortages while looking for alternate suppliers (see “Business – Production and Manufacturing for additional information).
We may not be able to replace our current manufacturing capabilities in a timely manner.
If our contract manufacturing facility or our in-house facility suffers any type of prolonged interruption, whether caused by regulator action, equipment failure, critical facility services failure, fire, natural disaster or any other event that causes the cessation of manufacturing activities, such as COVID-19, we may be exposed to long-term loss of sales and profits. There are limited facilities which are capable of contract manufacturing some of our products and product candidates. Replacement of our current manufacturing capabilities may have a material adverse effect on our business and financial condition.
We are dependent upon third-party service providers. If such third-party service providers fail to maintain a high quality of service, the utility of our products could be impaired, which could adversely affect the penetration of our products, our business, operating results and reputation.
The success of certain services and products that we provide are dependent upon third-party service providers. Such service providers include manufacturers of proprietary custom components for our ProSense and next generation single Probe and MultiSense systems. As we expand our commercial activities, an increased burden will be placed upon the quality of such third-party providers. If third-party providers fail to maintain a high quality of service, our products, business, reputation and operating results could be adversely affected. In addition, poor quality of service by third-party service providers could result in liability claims and litigation against us for damages or injuries.
18
If we are not able to attract and retain highly skilled managerial, scientific, technical and marketing personnel, we may not be able to implement our business model successfully.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. We are highly dependent upon our senior management as well as other employees, consultants and scientific and medical collaborators. Our management team must be able to act decisively to apply and adapt our business model in the rapidly changing markets in which we will compete. In addition, we will rely upon technical and scientific employees or third-party contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled managerial, sales, scientific and technical personnel. In order to do so, we may need to pay higher compensation or fees to our employees or consultants than currently expected and such higher compensation payments may have a negative effect on our operating results. Competition for experienced, high-quality personnel in the medical device field is intense. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain quality personnel on acceptable terms could impair our ability to develop new products and services and manage our business effectively.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks.
We may evaluate various acquisition opportunities and strategic partnerships, including licensing or acquiring complementary products, intellectual property rights, technologies or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
| ● | increased operating expenses and cash requirements; |
| ● | the assumption of additional indebtedness or contingent liabilities; |
| ● | the issuance of our equity securities; |
| ● | assimilation of operations, intellectual property and products of an acquired company, including difficulties associated with integrating new personnel; |
| ● | the diversion of our management’s attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition; |
| ● | retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships; |
| ● | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and marketing approvals; and |
| ● | our inability to generate revenues from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
We are subject to certain U.S. and foreign anticorruption, anti-money laundering, export control, sanctions and other trade laws and regulations. We can face serious consequences for violations.
Among other matters, U.S. and foreign anticorruption, anti-money laundering, export control, sanctions and other trade laws and regulations, which are collectively referred to as Trade Laws, prohibit companies and their employees, agents, clinical research organizations, legal counsel, accountants, consultants, contractors and other partners from authorizing, promising, offering, providing, soliciting or receiving, directly or indirectly, corrupt or improper payments or anything else of value to or from recipients in the public or private sector. Violations of Trade Laws can result in substantial criminal fines and civil penalties, imprisonment, the loss of trade privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. We also expect our non-U.S. activities to increase over time. We plan to engage third parties for clinical trials and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals, and we can be held liable for the corrupt or other illegal activities of our personnel, agents or partners, even if we do not explicitly authorize or have prior knowledge of such activities.
19
Non-U.S. governments often impose strict price controls, which may adversely affect our future profitability.
We may be subject to rules and regulations in the United States and non-U.S. jurisdictions relating to our ProSense and MultiSense systems or any future products. In some countries, including countries of the European Union, or the EU, Japan, or China each of which has developed its own rules and regulations, pricing may be subject to governmental control under certain circumstances. In these countries, pricing negotiations with governmental agencies can take considerable time after the receipt of marketing approval for a medical device candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available products. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
We manage our business through a small number of employees and key consultants.
As of July 6, 2021, we had 42 full-time employees, 11 part time employees 1 independent contractor and 2 consultants. Our future growth and success depend to a large extent on the continued services of members of our current management including, in particular, our VP Research and Development and our Chief Executive Officer. Any of our employees and consultants may leave our company at any time, subject to certain notice periods. The loss of the services of any of our executive officers or any key employees or consultants may adversely affect our ability to execute our business plan and harm our operating results. Our operational success will substantially depend on the continued employment of senior executives, technical staff and other key personnel. The loss of key personnel may have an adverse effect on our operations and financial performance
We may need to expand our organization and we may experience difficulties in recruiting needed additional employees and consultants, which could disrupt our operations.
As our development and commercialization plans and strategies develop and because we are leanly staffed, we may need additional managerial, development, operational, sales, marketing, financial, legal and other resources. The competition for qualified personnel in the medical device industry is intense. Due to this intense competition, we may be unable to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
Our management may need to divert its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional medical device products. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize medical device products and services and compete effectively will depend, in part, on our ability to effectively manage any future growth.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States or Israel.
Other than our headquarters and other operations which are located in Israel (as further described below), our business strategy incorporates significant international expansion, particularly in anticipated expansion of regulatory approvals of our products. Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; | |
| ● | failure by us to obtain regulatory approvals for the use of our products and services in various countries; | |
| ● | additional potentially relevant third-party patent rights; | |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; |
20
| ● | difficulties in staffing and managing foreign operations; | |
| ● | complexities associated with managing multiple regulatory, governmental and reimbursement regimes; | |
| ● | limits in our ability to penetrate international markets; | |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; | |
| ● | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; | |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and | |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, or the FCPA, its books and records provisions or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
We face intense competition in the market, and as a result we may be unable to effectively compete in our industry.
The major market players within the cancer cryoablation care market and our primary competitors in the United States and abroad include Boston Scientific and Siemens Healthineers. Some of these companies hold significant market share. Their dominant market position and significant control over the market could significantly limit our ability to introduce or effectively market and generate sales of our ProSense and MultiSense systems.
Many of our competitors have long histories and strong reputations within the industry. They have significantly greater brand recognition, financial and human resources than we do. They also have more experience and capabilities in researching and developing testing devices, obtaining and maintaining regulatory clearances and other requirements, manufacturing and marketing those products than we do. There is a significant risk that we may be unable to overcome the advantages held by our competition, and our inability to do so could lead to the failure of our business and the loss of your investment. In addition, we may be unable to develop additional products in the future or to keep pace with developments and innovations in the market and lose market share to our competitors.
Competition in the medical devices and cancer treatment market is intense, and can lead to, among other things, price reductions, longer selling cycles, lower product margins, loss of market share and additional working capital requirements. To succeed, we must, among other critical matters, gain consumer acceptance for our ProSense and next generation single Probe and MultiSense systems, as compared to other solutions currently available in the market for the treatment of tumors and potential future medical devices incorporating our principal technology or offering other advanced cryoablation, heat ablation or other non or minimal invasive solutions. For example, since the currently accepted treatment for breast cancer is surgery, we will need to invest resources in educating the medical community and consumers, and establish strategic collaborations before we able to gain market acceptance for our ProSense system as a treatment to breast cancer. If our competitors offer significant discounts on certain products and solutions, we may need to lower our prices or offer other favorable terms in order to compete successfully. Moreover, any broad-based changes to our prices and pricing policies could make it difficult to generate revenues or cause our revenues to decline. Moreover, if our competitors develop and commercialize products and solutions that are more effective or desirable than products and solutions that we may develop, we may not convince our customers to use our products and solutions. Any such changes would likely reduce our commercial opportunity and revenues potential and could materially adversely impact our operating results.
21
Our commercial success is very much dependent on third-party payors to provide adequate insurance coverage and reimbursement for the use of our systems, or any future products that we may commercialize.
Our ProSense and next generation single Probe and MultiSense systems, and any other product in our development pipeline, is not yet approved for third-party payor coverage or reimbursement in some of the geographical markets in which we operate, or plan to operate in the future. Such reimbursement may vary based on the particular device used in providing services and is based on the identity of the third-party. Our ability to maintain a leading position in the medical device market, and specifically in the cancer care market, depends on our relationships with private third parties.
We expect to engage with private third parties to allow our customers to receive reimbursement from insurance companies for our ProSense and next generation single Probe and MultiSense systems. The loss of a significant number of private third-party contracts may have an adverse effect on our revenues, which could have an adverse effect on our business, financial condition and results of operations. Over the past few years, reimbursement rates from certain third parties have declined, in some cases significantly. There can be no assurance that this trend will not continue or apply on more third parties.
In addition, private third parties may not reimburse any new procedures conducted with our products or reimburse those new clinical procedures at commercially viable rates. The failure to receive reimbursement at adequate levels for our existing or future products may adversely affect demand for those products, our revenues and expected growth. This could have an adverse effect on our business, financial condition and results of operations.
We may be subject to litigation for a variety of claims, which could adversely affect our results of operations, harm our reputation or otherwise negatively impact our business.
We may be subject to litigation for a variety of claims arising from our normal business activities. These may include claims, suits, and proceedings involving labor and employment, wage and hour, commercial and other matters. The outcome of any litigation, regardless of its merits, is inherently uncertain. Any claims and lawsuits, and the disposition of such claims and lawsuits, could be time-consuming and expensive to resolve, divert management attention and resources, and lead to attempts on the part of other parties to pursue similar claims. Any adverse determination related to litigation could adversely affect our results of operations, harm our reputation or otherwise negatively impact our business. In addition, depending on the nature and timing of any such dispute, a resolution of a legal matter could materially affect our future operating results, our cash flows and our ability to raise capital.
We could become subject to product liability, warranty or similar claims and product recalls that could be expensive, divert management’s attention and harm our business reputation and financial results.
Our business exposes us to an inherent risk of potential product liability, warranty or similar claims and product recalls. The medical device industry has historically been litigious, and we face financial exposure to product liability, warranty or similar claims if the use of any of our products were to cause or contribute to injury or death. There is also the possibility that defects in the design or manufacture of any of our products might necessitate a product recall. Although we plan to maintain product liability insurance, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration and a substantial diversion of management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations.
22
Our management team has limited experience managing a U.S. reporting company.
Most members of our management team do not have experience managing a publicly traded company in the United States, interacting with public company investors and complying with the increasingly complex laws pertaining to public companies in the United States. Although we are a public company in Israel, our management team may not successfully or efficiently manage our transition to being a public company in the United States that is subject to significant regulatory oversight and reporting obligations under the U.S. federal securities laws and the continuous scrutiny of securities analysts and investors. These new obligations and constituents will require significant attention from our senior management and could divert their attention away from the day-to-day management of our business, which could adversely affect our business, financial condition, results of operations and prospects.
Risks Related to Product Development and Regulatory Approval
Our product candidates and operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
We expect our ProSense, next generation single Probe and MultiSense systems and any future products we develop to be regulated by the FDA as medical devices. Regulation in the United States may subject us to the jurisdiction of the FDA, the U.S. Department of Justice, or the DOJ, and the U.S. Health and Human Services-Office of the Inspector General, or the HHS. Outside of the United States, we may be subject to the regulation of the FDA’s foreign counterparts as well as other foreign regulators. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance and approval; conformity assessment procedures; record keeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to occur, could lead to death or serious injury; post-market approval studies; and product import and export.
The regulations our product candidate is subject to are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales for any approved product. Failure to comply with applicable regulations could jeopardize our ability to sell our future products, if cleared or approved, and result in enforcement actions such as: warning or untitled letters; fines; injunctions; consent decrees; civil penalties; customer notifications; termination of distribution; recalls or seizures of products; administrative detention of medical devices believed to be adulterated or misbranded; delays in the introduction of products into the market; operating restrictions; total or partial suspension of production; refusal to grant future clearances or approvals for new products, new intended uses or modifications to our products; withdrawals or suspensions of current approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal prosecution or penalties. The occurrence of any of these events would have a material adverse effect on our business, financial condition and results of operations and could result in shareholders losing their entire investment.
Our, or our partners’, clinical trials may encounter delays, suspensions or other problems.
We, or our partners, may encounter problems in clinical trials that may cause us or the FDA or foreign regulatory agencies to delay, suspend or terminate any such clinical trials at any phase. These problems could include the possibility that we may not be able to conduct clinical trials at our preferred sites, enroll a sufficient number of patients for our clinical trials at one or more sites or begin or successfully complete clinical trials in a timely fashion, if at all. Furthermore, we, our partners, the FDA or foreign regulatory agencies may suspend clinical trials at any time if we or they believe the subjects participating in the trials are being exposed to unacceptable health risks or if we or they find deficiencies in the clinical trial process or conduct of the investigation. If clinical trials of any of our products fail, we will not be able to market the product which is the subject of the failed clinical trials. The FDA and foreign regulatory agencies could also require additional clinical trials, which would result in increased costs and significant development delays. Our, or our partners’, failure to adequately demonstrate the safety and effectiveness of a product under development could delay or prevent regulatory approval of the product and could have a material adverse effect on our business, prospects, financial condition and results of operations. Finally, the COVID-19 pandemic has impacted clinical trials broadly. We, or our partners, may experience delays in site initiation and patient enrollment, failures to comply with study protocols, delays in the manufacture of our product candidates for clinical testing and other difficulties in starting or competing our clinical trials, and we do not know if the existence of a vaccine for COVID-19 will have an impact on the potential occurrence of the risks that are associated with clinical trials as a result of COVID-19.
23
We may not receive, or may be delayed in receiving, the necessary clearances or approvals for our ProSense, next generation single Probe and MultiSense systems or future products in order to commercialize these products in specific countries or regions or in a specific indication, and failure to timely obtain necessary clearances or approvals for our existing or future products would adversely affect our ability to grow our business.
In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA, or De Novo classification or approval of a pre-market approval application, or a PMA, from the FDA, unless an exemption applies. In the 510(k)-clearance process, before a device may be marketed, the FDA must determine that a proposed device is “substantially equivalent” to a legally-marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre-amendments device), a device that was originally on the U.S. market pursuant to an approved PMA and later down-classified, or a 510(k)-exempt device. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence. The FDA may request clinical data in addition that provided from our clinical sites outside the United States. In the process of obtaining De Novo classification or PMA approval, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices.
Modifications to products that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made to products cleared through a 510(k)-clearance process may require a new 510(k) clearance. Both the PMA approval and the 510(k)-clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k)-clearance process usually takes from three to 12 months, but can last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k)-clearance process and generally takes from one to three years, or even longer, from the time the application is submitted to the FDA. In addition, a PMA generally requires the performance of one or more clinical trials. Despite the time, effort and cost, a device may not be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory clearances or approvals could harm our business. Furthermore, even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses for the device or other restrictions or requirements, which may limit the market for the device.
In the United States, we cleared the 510(k) application process and received regulatory approval to market our ProSense system and related accessories systems for the treatment of kidney and liver tumors. Specifically, FDA 510(k) approval covers IceSense3, ProSense, next generation single Probe and MultiSense systems and MultiSense, including the ancillary products thereto, such as Probes and ancillary products, and software updates. However, even after receiving this regulatory approval from the FDA, we require additional approvals from the FDA in order to begin commercialization efforts capable of generating significant revenues for us.
Our 510(k) application may not be cleared by the FDA in a timely manner or at all, in this case we will suggest submitting for De Novo classification and gain FDA agreement. If cleared, any modification to our ProSense system that has not been previously cleared for treatment of the indication for which approval was granted may require us to submit a new 510(k) premarket notification and obtain clearance, or submit a PMA and obtain FDA approval prior to implementing the change. Specifically, any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or, possibly, approval of a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. We may make modifications or add additional features in the future that we believe do not require a new 510(k) clearance or approval of a PMA. If the FDA disagrees with our determination and requires us to submit new 510(k) notifications or PMA applications for modifications to our previously cleared products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
24
The FDA can delay, limit or deny clearance or approval of a medical device for many reasons, including:
| ● | our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory entity or notified body that our product candidates are safe or effective for their intended uses; | |
| ● | the disagreement of the FDA or the applicable foreign regulatory body with the design or the interpretation of data from pre-clinical studies or clinical trials; | |
| ● | serious and unexpected adverse effects experienced by participants in our clinical trials; | |
| ● | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; | |
| ● | requesting clinical data from our trials at sites located outside of the United States; | |
| ● | our inability to demonstrate that the clinical and other benefits of the device outweigh the risks; | |
| ● | the manufacturing process or facilities we use may not meet applicable requirements; and | |
| ● | the potential for approval policies or regulations of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval. |
In order to sell our products in member countries of the European Economic Area, or EEA, our products must comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC) and with the Medical Device Regulations 2017/745 of the European Parliament and of the Council, which will fully enter into force on May 26, 2024. Compliance with these requirements is a prerequisite to be able to affix the Conformité Européene, or CE, mark to our products, without which they cannot be sold or marketed in the EEA. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can issue a European Community, or EC, Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the EU Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a member state of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE mark and the Notified Body number to its medical devices after having prepared and signed a related EC Declaration of Conformity.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. If we fail to remain in compliance with applicable European laws and directives, we would be unable to continue to affix the CE mark to our products, which would prevent us from selling them within the EEA.
25
Preliminary data that we or others announce or publish from time to time with respect to our products may change as more data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we, or our partners, may publish or seek to publish preliminary data from ongoing clinical trials, which are based on a preliminary analysis of then-available data. Positive preliminary data may not be predictive of such trial’s subsequent or overall results. Preliminary data are subject to the risk that one or more of the results and related findings and conclusions may materially change following a more comprehensive review of the data or as more data become available. Therefore, positive preliminary results in any ongoing clinical trial may not be predictive of such results in the completed trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully evaluate all data. As a result, preliminary data that we report may differ from future results from the same clinical trials, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, preliminary data should be viewed with caution until the final data are available. Material adverse changes in the final data compared to preliminary data could significantly harm our business prospects.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure. If the interim, top-line or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, in scale, our product candidates may be harmed, which could harm our business, operating results, prospects or financial condition.
Healthcare legislative and regulatory reform measures may have a material adverse effect on our business and results of operations.
Our industry is highly regulated and changes in law may adversely impact our business, operations or financial results. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the PPACA, is a sweeping measure intended to, among other things, expand healthcare coverage within the United States, primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. Several provisions of the law may affect us and increase certain of our costs.
In addition, other legislative changes have been adopted since the PPACA was enacted. These changes include aggregate reductions in Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, following passage of the Bipartisan Budget Act of 2018, will remain in effect through 2027 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and, accordingly, our financial operations.
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the reimbursement our customers may receive for our products. Further, there have been, and there may continue to be, judicial and Congressional challenges to certain aspects of the PPACA. For example, the U.S. Tax Cuts and Jobs Act of 2017, or TCJA, includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Additional legislative and regulatory changes to the PPACA, its implementing regulations and guidance and its policies, remain possible in Congress and under the Trump administration. However, it remains unclear how any new legislation or regulation might affect the prices we may obtain for any of our products for which regulatory approval is obtained. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate significant revenue, attain profitability or commercialize our products in scale.
26
In addition, the delivery of healthcare in the European Union, including the establishment and operation of health services, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay additional marketing approval of our ProSense system or any initial marketing approval for our ProSense system or any future product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval.
We are currently unable to predict what additional legislation or regulation, if any, relating to the health care industry may be enacted in the future or what effect recently enacted federal legislation or any such additional legislation or regulation would have on our business. The pendency or approval of such proposals or reforms could result in a decrease in the price of our Ordinary Shares or limit our ability to raise capital or to enter into collaboration agreements for the further development and potential commercialization of our products.
Failure to comply with post-marketing regulatory requirements could subject us to enforcement actions, including substantial penalties, and might require us to recall or withdraw a product from the market.
Although we have certain regulatory approvals to market our ProSense system, we are still subject to ongoing and pervasive regulatory requirements governing, among other things, the manufacture, marketing, advertising, medical device reporting, sale, promotion, import, export, registration, and listing of devices. In addition, if we receive additional regulatory approvals to market the ProSense system or regulatory approvals to market the MultiSense system or other products we will likewise remain subject to ongoing regulation. For example, we will be required to submit periodic reports to the FDA as a condition of 510(k) clearance, which we have received for our ProSense system and related accessories, for the treatment of kidney and liver tumors. These reports include information about failures and certain adverse events associated with the device after its clearance. Failure to submit such reports, or failure to submit the reports in a timely manner, could result in enforcement action by the FDA. Following its review of the periodic reports, the FDA might ask for additional information or initiate further investigation.
The regulations to which we are subject are complex and have become more stringent over time. Regulatory changes could result in restrictions on our ability to continue or expand our operations, higher than anticipated costs, or lower than anticipated sales. Even after we have obtained the proper regulatory clearance to market a device, we have ongoing responsibilities under FDA regulations and applicable foreign laws and regulations. The FDA, state and foreign regulatory authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA, state or foreign regulatory authorities, which may include any of the following sanctions:
| ● | untitled letters or warning letters; | |
| ● | fines, injunctions, consent decrees and civil penalties; | |
| ● | recalls, termination of distribution, administrative detention, or seizure of our products; | |
| ● | customer notifications or repair, replacement or refunds; | |
| ● | operating restrictions or partial suspension or total shutdown of production; | |
| ● | delays in or refusal to grant our requests for future clearances or approvals or foreign marketing authorizations of new products, new intended uses, or modifications to existing products; | |
| ● | withdrawals or suspensions of product clearances or approvals, resulting in prohibitions on sales of our products; | |
| ● | FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; and | |
| ● | criminal prosecution. |
27
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, financial condition and results of operations.
In addition, the FDA or state or foreign authorities may change their clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay clearance or approval of our future products under development on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain new clearances or approvals, increase the costs of compliance or restrict our ability to maintain any approvals we are able to obtain. For example, the FDA recently announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the FDCA.
Our products must be manufactured in accordance with federal, state and foreign regulations, and we could be forced to recall our devices or terminate production if we fail to comply with these regulations.
The methods used in, and the facilities used for, the manufacture of our products must comply with the Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of medical devices. As manufacturers of electron radiation-emitting products, we are also responsible for compliance with the radiological health regulations and certain radiation safety performance standards.
Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. Our products are also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing.
Our third-party manufacturers may not take the necessary steps to comply with applicable regulations, which could cause delays in the delivery of our products. In addition, failure to comply with applicable FDA or state or foreign requirements or later discovery of previously unknown problems with our products or manufacturing processes could result in, among other things: warning letters or untitled letters; fines, injunctions or civil penalties; suspension or withdrawal of approvals; seizures or recalls of our products; total or partial suspension of production or distribution; administrative or judicially imposed sanctions; the FDA’s refusal to grant pending or future clearances or approvals for our products; clinical holds; refusal to permit the import or export of our products; and criminal prosecution of us, our suppliers or our employees.
Any of these actions could significantly and negatively affect supply of our products. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we could lose customers and experience reduced sales and increased costs.
Current and future legislation may increase the difficulty and cost for us and collaborators, if any, to obtain marketing approval of and commercialize, in scale, our products and affect the prices we, or they, may obtain.
In the United States and some foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system, including cost-containment measures that may reduce or limit coverage and reimbursement for newly approved drugs or medical procedures and affect our ability to profitably sell any product candidates for which we obtain marketing approval. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare.
For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, was enacted in the United States. Among the provisions of the Affordable Care Act of importance to our potential product candidates, the Affordable Care Act established an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; expands eligibility criteria for Medicaid programs; increased the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; created a new Medicare Part D coverage gap discount program; required certain Affordable Care Act marketplace and other private payor plans to include coverage for preventative services, including vaccinations recommended by the ACIP without cost share obligations (i.e., co-payments, deductibles or co-insurance) for plan members; established a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research; and established a Center for Medicare and Medicaid Innovation at the Centers for Medicare & Medicaid Services, or CMS, to test innovative payment and service delivery models to lower Medicare and Medicaid spending.
28
Since its enactment, there have been judicial and congressional challenges to numerous aspects of the Affordable Care Act. By way of example, the 2017 Tax Reform Act included a provision repealing the individual mandate, effective January 1, 2019. On December 14, 2018, a U.S. District Court judge in the Northern District of Texas ruled that the individual mandate portion of the Affordable Care Act is an essential and inseverable feature of the Affordable Care Act, and therefore because the mandate was repealed, the remaining provisions of the Affordable Care Act are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the Fifth Circuit upheld the District Court ruling that the individual mandate was unconstitutional, but remanded the case back to the District Court to determine whether the remaining provisions of the Affordable Care Act are invalid as well. On March 2, 2020, the U.S. Supreme Court granted the petitions for writs of certiorari to review the case, although it is unclear when a decision will be made or how the U.S. Supreme Court will rule. In addition, there may be other efforts to challenge, repeal or replace the Affordable Care Act. We are continuing to monitor any changes to the Affordable Care Act that, in turn, may potentially impact our business in the future.
Other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. These changes include aggregate reductions to Medicare payments to providers of 2% per fiscal year pursuant to the Budget Control Act of 2011 and subsequent laws, which began in 2013 and will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through December 31, 2020 under the Coronavirus Aid, Relief and Economic Security Act, unless additional Congressional action is taken. In addition, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. New laws may result in additional reductions in Medicare and other healthcare funding, which may materially adversely affect customer demand and affordability for our product candidates, if approved, and, accordingly, the results of our financial operations.
We cannot predict whether future healthcare legislative or policy changes will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us, but we expect there will continue to be legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care.
The misuse or off-label use of our products may harm our reputation in the marketplace, result in injuries that may lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
Advertising and promotion of our products that obtain marketing approval in the United States may be heavily scrutinized by the FDA, the DOJ, HHS, state attorneys general, members of Congress, and the public. In addition, advertising and promotion of any product that obtains approval outside of the United States may be heavily scrutinized by comparable foreign regulatory authorities.
We expect that, if cleared or approved, our products, will be cleared by the requisite regulatory authorities for specific indications. We expect to train our marketing personnel and direct sales force to not promote our devices for uses outside of the FDA-approved indications for use, known as “off-label uses.” We cannot, however, prevent a physician from using our devices off-label, when in the physician’s independent professional medical judgment, he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our devices off-label. Furthermore, the use of our devices for indications other than those approved by the FDA or approved by any foreign regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among healthcare providers and patients.
If the FDA or any state or foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of an untitled letter, which is used for violators that do not necessitate a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations. We may become subject to such actions and, if we are not successful in defending against such actions, those actions may have a material adverse effect on our business, financial condition and results of operations. Equivalent laws and potential consequences exist in foreign jurisdictions.
29
In addition, if our products are cleared or approved, healthcare providers may misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If our devices are misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. As described above, product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance.
Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to immediately report to all relevant regulatory authorities, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
We are subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of the product. If we fail to comply with our reporting obligations, the FDA or other regulatory bodies could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device clearance or approval, seizure of our products or delay in clearance or approval of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. Product defects or other errors may occur in the future.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new clearances or approvals for the device before we may market or distribute the corrected device. Seeking such clearances or approvals may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
30
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Many federal, state and foreign healthcare laws and regulations apply to medical devices. We may be subject to certain federal and state regulations, including the federal healthcare programs’ Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, offering, receiving, or paying any remuneration, directly or indirectly, in cash or in kind, to induce or reward purchasing, ordering or arranging for or recommending the purchase or order of any item or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services; the federal Civil Monetary Penalties Law, which authorizes the imposition of substantial civil monetary penalties against an entity that engages in activities including, among others (1) knowingly presenting, or causing to be presented, a claim for services not provided as claimed or that is otherwise false or fraudulent in any way; (2) arranging for or contracting with an individual or entity that is excluded from participation in federal healthcare programs to provide items or services reimbursable by a federal healthcare program; (3) violations of the federal Anti-Kickback Statute; or (4) failing to report and return a known overpayment; the federal False Statements Statute, which prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry, in connection with the delivery of or payment for healthcare benefits, items, or services; the federal civil False Claims Act, or the FCA, which prohibits, among other things, knowingly presenting, or causing to be presented claims for payment of government funds that are false or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to such a false or fraudulent claim, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government; and other federal and state false claims laws. The FCA prohibits anyone from knowingly presenting, conspiring to present, making a false statement in order to present, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. This law also prohibits anyone from knowingly underpaying an obligation owed to a federal program. Increasingly, U.S. federal agencies are requiring nonmonetary remedial measures, such as corporate integrity agreements in FCA settlements. The DOJ announced in 2016 its intent to follow the “Yates Memo,” taking a far more aggressive approach in pursuing individuals as FCA defendants in addition to corporations.
The majority of states also have statutes similar to the federal Anti-Kickback Statute and false claims laws that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, that apply regardless of whether the payer is a government entity or a private commercial entity. The Federal Open Payments, or Physician Payments Sunshine Act, program requires manufacturers of products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program, to track and report annually to the federal government (for disclosure to the public) certain payments and other transfers of value made to physicians and teaching hospitals as well as disclosure of payments and other transfers of value provided to physicians and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations. Our failure to appropriately track and report payments to the government could result in civil fines and penalties, which could adversely affect the results of our operations. In addition, several U.S. states and localities have enacted legislation requiring medical device companies to establish marketing compliance programs, file periodic reports with the state, and/or make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities. Other state laws prohibit certain marketing-related activities including the provision of gifts, meals or other items to certain healthcare providers. Many of these laws and regulations contain ambiguous requirements that government officials have not yet clarified. Given the lack of clarity in the laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent federal and state laws and regulations.
The medical device industry has been under heightened scrutiny as the subject of government investigations and enforcement actions involving manufacturers who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business, including arrangements with physician consultants. If our operations or arrangements are found to be in violation of such governmental regulations, we may be subject to civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment of our operations. All of these penalties could adversely affect our ability to operate our business and our financial results.
31
Changes in laws or regulations relating to data protection, or any actual or perceived failure by us to comply with such laws and regulations or our privacy policies, could materially and adversely affect our business or could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
We expect to receive health information and other highly sensitive or confidential information and data of patients and other third parties (e.g., healthcare providers who refer patients for scans), which we expect to compile and analyze. Collection and use of this data might raise privacy and data protection concerns, which could negatively impact our business. There are numerous federal, state and international laws and regulations regarding privacy, data protection, information security, and the collection, storing, sharing, use, processing, transfer, disclosure, and protection of personal information and other data, and the scope of such laws and regulations may change, be subject to differing interpretations, and may be inconsistent among countries and regions we intend to operate in (e.g., the United States, the European Union and Israel), or conflict with other laws and regulations. The regulatory framework for privacy and data protection worldwide is, and is likely to remain for the foreseeable future, uncertain and complex, and this or other actual or alleged obligations may be interpreted and applied in a manner that we may not anticipate or that is inconsistent from one jurisdiction to another and may conflict with other rules or practices including ours. Further, any significant change to applicable laws, regulations, or industry practices regarding the collection, use, retention, security, or disclosure of data, or their interpretation, or any changes regarding the manner in which the consent of relevant users for the collection, use, retention, or disclosure of such data must be obtained, could increase our costs and require us to modify our services and candidate products, possibly in a material manner, which we may be unable to complete, and may limit our ability to store and process patients’ data or develop new services and features.
In particular, we will be subject to U.S. data protection laws and regulations (i.e., laws and regulations that address privacy and data security) at both the federal and state levels. The legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on privacy and data security issues. Numerous federal and state laws, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws, govern the collection, use, and disclosure of health-related and other personal information. Failure to comply with such laws and regulations could result in government enforcement actions and create liability for us (including the imposition of significant civil or criminal penalties), private litigation and/or adverse publicity that could negatively affect our business. For instance, California enacted the California Consumer Privacy Act (CCPA) on June 28, 2018, which took effect on January 1, 2020. The CCPA creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal data. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability, and many similar laws have been proposed at the federal level and in other states.
In addition, we expect to obtain health information that is subject to privacy and security requirements under the Health Information Technology for Economic and Clinical Health, or HITECH, and its implementing regulations. The Privacy Standards and Security Standards under HIPAA establish a set of standards for the protection of individually identifiable health information by health plans, health care clearinghouses and certain health care providers, referred to as Covered Entities, and the business associates with whom Covered Entities enter into service relationships pursuant to which individually identifiable health information may be exchanged. Notably, whereas HIPAA previously directly regulated only Covered Entities, HITECH makes certain of HIPAA’s privacy and security standards also directly applicable to Covered Entities’ business associates. As a result, both Covered Entities and business associates are now subject to significant civil and criminal penalties for failure to comply with Privacy Standards and Security Standards. As part of our normal operations, we expect to collect, process and retain personal identifying information regarding patients, including as a business associate of Covered Entities, so we expect to be subject to HIPAA, including changes implemented through HITECH, and we could be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information in a manner that is not authorized or permitted by HIPAA. A data breach affecting sensitive personal information, including health information, also could result in significant legal and financial exposure and reputational damages that could potentially have an adverse effect on our business.
HIPAA requires Covered Entities (like many of our potential customers) and business associates, like us, to develop and maintain policies and procedures with respect to protected health information that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information. HITECH expands the notification requirement for breaches of patient-identifiable health information, restricts certain disclosures and sales of patient-identifiable health information and provides for civil monetary penalties for HIPAA violations. HITECH also increased the civil and criminal penalties that may be imposed against Covered Entities and business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA and its implementing regulations and seek attorney’s fees and costs associated with pursuing federal civil actions. Additionally, certain states have adopted comparable privacy and security laws and regulations, some of which may be more stringent than HIPAA.
32
Internationally, many jurisdictions have or are considering enacting privacy or data protection laws or regulations relating to the collection, use, storage, transfer, disclosure and/or other processing of personal data, as well as certification requirements for the hosting of health data specifically. Such laws and regulations may include data hosting, data residency or data localization requirements (which generally require that certain types of data collected within a certain country be stored and processed within that country), data export restrictions, international transfer laws (which prohibit or impose conditions upon the transfer of such data from one country to another), or may require companies to implement privacy or data protection and security policies, enable users to access, correct and delete personal data stored or maintained by such companies, inform individuals of security breaches that affect their personal data or obtain individuals’ consent to use their personal data. For example, European legislators adopted the EU’s General Data Protection Regulation (2016/679), or GDPR, which became effective on May 25, 2018, and are now in the process of finalizing the ePrivacy Regulation to replace the European ePrivacy Directive (Directive 2002/58/EC as amended by Directive 2009/136/EC). The GDPR, supplemented by national laws and further implemented through binding guidance from the European Data Protection Board, imposes more stringent EU data protection requirements and provides for significant penalties for noncompliance. Further, the United Kingdom’s initiating a process to leave the EU has created uncertainty with regard to the regulation of data protection in the United Kingdom. In particular, the United Kingdom has brought the GDPR into domestic law with the Data Protection Act 2018 which will remain in force, even if and when the United Kingdom leaves the EU.
Virtually every jurisdiction in which we expect to operate has established its own data security and privacy legal framework with which we must, and our target customers will need to, comply, including the rules and regulation mentioned above. We may also need to comply with varying and possibly conflicting privacy laws and regulations in other jurisdictions. As a result, we could face regulatory actions, including significant fines or penalties, adverse publicity and possible loss of business.
While we are preparing to implement various measures intended to enable us to comply with applicable privacy or data protection laws, regulations and contractual obligations, these measures may not always be effective and do not guarantee compliance. Any failure or perceived failure by us to comply with our contractual or legal obligations or regulatory requirements relating to privacy, data protection, or information security may result in governmental investigations or enforcement actions, litigation, claims, or public statements against us by consumer advocacy groups or others and could result in significant liability, cause our customers, partners or patients to lose trust in us, and otherwise materially and adversely affect our reputation and business. Furthermore, the costs of compliance with, and other burdens imposed by, the laws, regulations, and policies that are applicable to the businesses of our customers or partners may limit the adoption and use of, and reduce the overall demand for, our products and services. Additionally, if third parties we work with violate applicable laws, regulations, or agreements, such violations may put the data we have received at risk, could result in governmental investigations or enforcement actions, fines, litigation, claims, or public statements against us by consumer advocacy groups or others and could result in significant liability, cause our customers, partners or patients to lose trust in us, and otherwise materially and adversely affect our reputation and business. Further, public scrutiny of, or complaints about, technology companies or their data handling or data protection practices, even if unrelated to our business, industry or operations, may lead to increased scrutiny of technology companies, including us, and may cause government agencies to enact additional regulatory requirements, or to modify their enforcement or investigation activities, which may increase our costs and risks.
If we do not obtain and maintain international regulatory registrations, clearances or approvals for our products, we will be unable to market and sell our products.
Sales of our products are subject to foreign regulatory requirements that vary widely from country to country. Approval procedures vary among countries and can involve additional testing. The time required to obtain approvals may differ substantially. While the regulations of some countries may not impose barriers to marketing and selling our products or only require notification, others require that we obtain the clearance or approval of a specified regulatory body. Complying with foreign regulatory requirements, including obtaining registrations, clearances or approvals, can be expensive and time-consuming, and we may not receive regulatory clearances or approvals in each country in which we plan to market our products or we may be unable to do so on a timely basis. The time required to obtain registrations, clearances or approvals, if required by other countries, may be longer than that required for FDA clearance or approval, and requirements for such registrations, clearances or approvals may significantly differ from FDA requirements. If we modify our products, we may need to apply for additional regulatory clearances or approvals before we are permitted to sell the modified product. In addition, we may not continue to meet the quality and safety standards required to maintain the authorizations that we have received. If we are unable to maintain our authorizations in a particular country, we will no longer be able to sell the applicable product in that country.
33
Regulatory clearance or approval by the FDA does not ensure registration, clearance or approval by regulatory authorities in other countries, and registration, clearance or approval by one or more foreign regulatory authorities does not ensure registration, clearance or approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining registration or regulatory clearance or approval in one country may have a negative effect on the regulatory process in others.
Legislative or regulatory reforms in the United States or the EU may make it more difficult and costly for us to obtain regulatory clearances or approvals for our products or to manufacture, market or distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. Over the last several years, the FDA has proposed reforms to its 510(k)-clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k)-clearance process for their products. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) clearance pathway toward the use of newer predicates. These proposals included plans to potentially sunset certain older devices that were used as predicates under the 510(k)-clearance pathway, and to potentially publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. In May 2019, the FDA solicited public feedback on these proposals. The FDA requested public feedback on whether it should consider certain actions that might require new authority, such as whether to sunset certain older devices that were used as predicates under the 510(k) clearance pathway. These proposals have not yet been finalized or adopted, and the FDA may work with Congress to implement such proposals through legislation. Accordingly, it is unclear the extent to which any proposals, if adopted, could impose additional regulatory requirements on us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance, or restrict our ability to maintain our current clearances, or otherwise create competition that may negatively affect our business.
More recently, in September 2019, the FDA finalized guidance describing an optional “safety and performance based” premarket review pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k) clearance pathway by showing that such device meets objective safety and performance criteria established by the FDA, thereby obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. The FDA intends to develop and maintain a list device types appropriate for the “safety and performance based” pathway and will continue to develop product-specific guidance documents that identify the performance criteria for each such device type, as well as the testing methods recommended in the guidance documents, where feasible. The FDA may establish performance criteria for classes of devices for which we or our competitors seek or currently have received clearance, and it is unclear the extent to which such performance standards, if established, could impact our ability to obtain new 510(k) clearances or otherwise create competition that may negatively affect our business.
In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain clearance or approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require: additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be promulgated that could prevent, limit or delay regulatory clearance or approval of our future products. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. For example, certain policies of the Trump administration may impact our business and industry. Namely, the Trump administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, FDA’s ability to engage in routine oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. It is difficult to predict how these executive actions will be implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority. If these executive actions impose restrictions on the FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval or clearance that we may have obtained and we may not achieve or sustain profitability.
34
On April 5, 2017, the European Parliament passed the Medical Devices Regulation (Regulation 2017/745), which repeals and replaces the EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations would be directly applicable, i.e., without the need for adoption of EEA member state laws implementing them, in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation. Among other things, the Medical Devices Regulation:
| ● | strengthen the rules on placing devices on the market and reinforce surveillance once they are available; | |
| ● | establish explicit provisions on manufacturers’ responsibilities for follow-up regarding the quality, performance and safety of devices placed on the market; | |
| ● | improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; | |
| ● | set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and | |
| ● | strengthened rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts before they are placed on the market. |
These modifications may have an effect on the way we conduct our business in the EEA.
Disruptions at the FDA and other government agencies could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being cleared or approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA to review and clear or approve new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory, and policy changes, the FDA’s ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new medical devices or modifications to cleared or approved medical devices to be reviewed and/or approved by necessary government agencies, which would adversely affect our business.
Separately, in response to the COVID-19 pandemic, on March 10, 2020 the FDA announced its intention to postpone most inspections of foreign manufacturing facilities, and on March 18, 2020, the FDA temporarily postponed routine surveillance inspections of domestic manufacturing facilities. Subsequently, on July 10, 2020 the FDA announced its intention to resume certain on-site inspections of domestic manufacturing facilities subject to a risk-based prioritization system. The FDA intends to use this risk-based assessment system to identify the categories of regulatory activity that can occur within a given geographic area, ranging from mission critical inspections to resumption of all regulatory activities. Regulatory authorities outside the United States may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
35
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain effective patent rights for our products and services, we may not be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us.
Our success and future revenues growth will depend, in part, on our ability to protect our patent rights. In addition to the protection afforded by any patents that may be granted, historically, we have relied on trade secret protection and confidentiality agreements with our employees, consultants, and contractors to protect proprietary know-how that is not patentable or that we elect not to patent, processes that are not easily known, knowable or easily ascertainable, and for which patent infringement is difficult to monitor and enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, agreements may be breached, trade secrets may be difficult to protect, and we may not receive adequate remedies for any breach. In addition, our trade secrets and intellectual property may otherwise become known or be independently discovered by competitors or other unauthorized third parties.
There is no guarantee that the patent registration applications that we submitted with regards to our technologies will result in patent registration. In the event of failure to complete patent registration, our developments will not be proprietary, which might allow other entities to manufacture our products or design our services and compete with them.
Further, there is no assurance that all potentially relevant prior art relating to our patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our products or services, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patent applications and any future patents may not adequately protect our intellectual property, products or services and provide exclusivity for our new products or services or prevent others from designing around our claims. Furthermore, there is no guarantee that third parties will not infringe or misappropriate our patents or similar proprietary rights. In addition, there can be no assurance that we will not have to pursue litigation against other parties to assert its rights.
Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
If we cannot obtain and maintain effective patent rights for our products and services, we may not be able to compete effectively, and our business and results of operations would be harmed.
We cannot provide any assurances that our trade secrets and other confidential proprietary information will not be disclosed in violation of our confidentiality agreements or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Also, misappropriation or unauthorized and unavoidable disclosure of our trade secrets and intellectual property could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets and intellectual property are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secret.
Intellectual property rights of third parties could adversely affect our ability to commercialize our products and services, and we might be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation or licenses could be costly or not available on commercially reasonable terms.
It is inherently difficult to conclusively assess our freedom to operate without infringing on third-party rights. Our competitive position may be adversely affected if existing patents or patents resulting from patent applications issued to third parties or other third-party intellectual property rights are held to cover our products or services or elements thereof, or our manufacturing or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize products or services or our product candidates (and any relevant services) unless we successfully pursue litigation to nullify or invalidate the third-party intellectual property right concerned or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms. There may also be pending patent applications that if they result in issued patents, could be alleged to be infringed by our new products or services. If such an infringement claim should be brought and be successful, we may be required to pay substantial damages, be forced to abandon our new products or services or seek a license from any patent holders. No assurances can be given that a license will be available on commercially reasonable terms, if at all.
36
It is also possible that we have failed to identify relevant third-party patents or applications. For example, U.S. patent applications filed before November 29, 2000 and certain U.S. patent applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our new products or services could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our services, our new products or the use of our new products. Third-party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in pursuing the development of and/or marketing our new products or services. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing our new products or services that are held to be infringing. We might, if possible, also be forced to redesign our new products so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing new products and services. As our industries expand and more patents are issued, the risk increases that our products and services may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, designs or methods of manufacture related to the use or manufacture of our products or services. There may be currently pending patent applications or continued patent applications that may later result in issued patents that our products or services may infringe. In addition, third parties may obtain patents or services in the future and claim that use of our technologies infringes upon these patents.
If any third-party patents were held by a court of competent jurisdiction to cover aspects of our processes for designs, or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtain a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our products or services. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or services, or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of any patents that may issue from our patent applications or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we were the first to file the invention claimed in our owned and licensed patent or pending applications, or that we or our licensor were the first to file for patent protection of such inventions. Assuming all other requirements for patentability are met, in the United States prior to March 15, 2013, the first to make the claimed invention without undue delay in filing, is entitled to the patent, while generally outside the United States, the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, enacted on September 16, 2011, the United States has moved to a first to file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. In general, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents, all of which could have a material adverse effect on our business and financial condition.
37
We may be involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe our intellectual property. If we were to initiate legal proceedings against a third-party to enforce a patent covering one of our new products or services, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the United States Patent and Trademark Office, or USPTO, or made a misleading statement, during prosecution. Under the Leahy-Smith Act, the validity of U.S. patents may also be challenged in post-grant proceedings before the USPTO. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Derivation proceedings initiated by third parties or brought by us may be necessary to determine the priority of inventions and/or their scope with respect to our patent or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our new products or services to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our Ordinary Shares.
We may be subject to claims challenging the inventorship of our intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in, or right to compensation, with respect to our current patent and patent applications, future patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our products or services. Litigation may be necessary to defend against these and other claims challenging inventorship or claiming the right to compensation. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on products and services, as well as monitoring their infringement in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States.
Competitors may use our technologies develop their own products or services in jurisdictions where we have not obtained patent protection to and may export infringing products or services to territories where we have patent protection, but where patents are not enforced as strictly as they are in the United States. These products or services may compete with our products or services. Future patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
38
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, which could make it difficult for us to stop the marketing of competing products or services in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our future patents at risk of being invalidated or interpreted narrowly, put the issuance of our patent applications at risk, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and any damages or other remedies that we may be awarded, may not be commercially meaningful. Accordingly, our efforts to monitor and enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to the Ownership of our Ordinary Shares
Future sales of our Ordinary Shares could reduce the market price of our Ordinary Shares.
Substantial sales of our Ordinary Shares on the Nasdaq Capital Market, including following this offering, may cause the market price of our Ordinary Shares to decline. Sales by our security holders of substantial amounts of our Ordinary Shares, or the perception that these sales may occur in the future, could cause a reduction in the market price of our Ordinary Shares.
The issuance of any additional Ordinary Shares or any securities that are exercisable for or convertible into Ordinary Shares, may have an adverse effect on the market price of our Ordinary Shares and will have a dilutive effect on our existing shareholders and holders of Ordinary Shares.
Our principal shareholders, officers and directors currently beneficially own approximately 75.81% of our Ordinary Shares. They will therefore be able to exert significant control over matters submitted to our shareholders for approval.
As of the date of this prospectus, our principal shareholders, officers and directors beneficially own approximately 75.81% of our Ordinary Shares. This significant concentration of share ownership may adversely affect the trading price for our Ordinary Shares because investors often perceive disadvantages in owning shares in companies with controlling shareholders. As a result, these shareholders, if they acted together, could significantly influence or even unilaterally approve matters requiring approval by our shareholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these shareholders may not always coincide with our interests or the interests of other shareholders.
We do not know whether a market for the Ordinary Shares will be sustained or what the trading price of the Ordinary Shares will be and as a result it may be difficult for you to sell your Ordinary Shares.
Although we intend to list the Ordinary Shares on Nasdaq, an active trading market for the Ordinary Shares may not be sustained. It may be difficult for you to sell your Ordinary Shares without depressing the market price for the Ordinary Shares or at all. As a result of these and other factors, you may not be able to sell your Ordinary Shares at or above the offering price or at all. Further, an inactive market may also impair our ability to raise capital by selling Ordinary Shares and may impair our ability to enter into strategic partnerships or acquire companies, products, or services by using our equity securities as consideration.
We have never paid cash dividends on our share capital, and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends, and we do not anticipate paying cash dividends in the foreseeable future. Therefore, you should not rely on an investment in Ordinary Shares as a source for any future dividend income. Our board of directors has complete discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors.
39
We may need additional capital, and the sale of additional shares or equity or debt securities could result in additional dilution to our stockholders.
We may need to raise additional capital through a combination of private and public equity offerings, debt financings and collaborations, and strategic and licensing arrangements. To the extent that we raise additional capital through the issuance of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a holder of our Ordinary Shares. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We may be a “passive foreign investment company,” or PFIC, for U.S. federal income tax purposes in the current taxable year or may become one in any subsequent taxable year. There generally would be negative tax consequences for U.S. taxpayers that are holders of the Ordinary Shares if we are or were to become a PFIC.
Based on the projected composition of our income and valuation of our assets, we do not expect to be a PFIC for 2021, and we do not expect to become a PFIC in the future, although there can be no assurance in this regard. The determination of whether we are a PFIC is made on an annual basis and will depend on the composition of our income and assets from time to time. We will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (1) at least 75% of our gross income is “passive income” or (2) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of the Ordinary Shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC in the future. If we are a PFIC in any taxable year during which a U.S. taxpayer holds the Ordinary Shares, such U.S. taxpayer would be subject to certain adverse U.S. federal income tax rules. In particular, if the U.S. taxpayer did not make an election to treat us as a “qualified electing fund”, or QEF, or make a “mark-to-market” election, then “excess distributions” to the U.S. taxpayer, and any gain realized on the sale or other disposition of the Ordinary Shares by the U.S. taxpayer: (1) would be allocated ratably over the U.S. taxpayer’s holding period for the Ordinary Shares; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the U.S. Internal Revenues Service, or the IRS, determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. taxpayer to make a timely QEF or mark-to-market election. U.S. taxpayers that have held the Ordinary Shares during a period when we were a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. taxpayer who made a timely QEF or mark-to-market election. A U.S. taxpayer can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. We do not intend to notify U.S. taxpayers that hold the Ordinary Shares if we believe we will be treated as a PFIC for any taxable year in order to enable U.S. taxpayers to consider whether to make a QEF election. In addition, we do not intend to furnish such U.S. taxpayers annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. U.S. taxpayers that hold the Ordinary Shares are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election with respect to the Ordinary Shares in the event that we are a PFIC (see “Taxation—U.S. Federal Income Tax Considerations—Passive Foreign Investment Companies” for additional information).
40
Risks Related to Israeli Law and Our Operations in Israel
Potential political, economic and military instability in the State of Israel, where our headquarters, members of our management team, our production and research and development facilities are located, may adversely affect our results of operations.
Our executive offices, research and development laboratories and manufacturing facility are located in Caesarea, Israel. In addition, the majority of our key employees, officers and directors are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and groups in its neighboring countries, Hamas (an Islamist militia and political group that has historically controlled the Gaza Strip) and Hezbollah (an Islamist militia and political group based in Lebanon). In addition, several countries, principally in the Middle East, restrict doing business with Israel, and additional countries may impose restrictions on doing business with Israel and Israeli companies whether as a result of hostilities in the region or otherwise. Any hostilities involving Israel, terrorist activities, political instability or violence in the region or the interruption or curtailment of trade or transport between Israel and its trading partners could adversely affect our operations and results of operations and the market price of our Ordinary Shares.
Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or, if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business, financial condition and results of operations.
Further, many Israeli citizens are obligated to perform several days, and in some cases more, of annual military reserve duty each year until they reach the age of 40 (or older for certain reservists) and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be military reserve duty call-ups in the future. Our operations could be disrupted by such call-ups, which may include the call-up of members of our management. Such disruption could materially adversely affect our business, prospects, financial condition and results of operations.
We expect to be exposed to fluctuations in currency exchange rates, which could adversely affect our results of operations.
We incur expenses in NIS, U.S. dollars, Euros and Chinese Yuan (CNY), but our financial statements are denominated in U.S. dollars. Accordingly, we face exposure to adverse movements in currency exchange rates. Our revenues in the EU is denominated in Euros. Accordingly, we face exposure to adverse movements in the currency exchange rate of the U.S. dollars against the Euro, which may have a negative effect on our revenue. If the U.S. dollar strengthens against the Euro, the translation of these foreign currency denominated transactions will result in decreased revenues in U.S. dollars. Our cost of operations in Israel and in China are influenced by any movements in the currency exchange rate of the NIS and CNY. Such movements in the currency exchange rate may have a negative effect on our financial results. If the U.S. dollar weakens against the NIS and CNY, the translation of the NIS and CNY currency denominated transactions to U.S. dollar will result in increased operating expenses. Similarly, if the U.S. dollar strengthens against NIS and CNY, the translation of these NIS and CNY denominated transactions to U.S. dollars will result in decreased expenses. As exchange rates vary, sales and other operating results, when translated, may differ materially from our or the capital market’s expectations.
The termination or reduction of tax and other incentives that the Israeli government provides to Israeli companies may increase our costs and taxes.
The Israeli government currently provides tax and capital investment incentives to Israeli companies, as well as grant and loan programs relating to research and development and marketing and export activities. In recent years, the Israeli government has reduced the benefits available under these programs and the Israeli governmental authorities may in the future further reduce or eliminate the benefits of these programs. We may take advantage of these benefits and programs in the future; however, there can be no assurance that such benefits and programs will be available to us. If we qualify for such benefits and programs and fail to meet the conditions thereof, the benefits could be canceled and we could be required to refund any benefits we might already have enjoyed and become subject to penalties. Additionally, if we qualify for such benefits and programs and they are subsequently terminated or reduced, it could have an adverse effect on our financial condition and results of operations.
41
We may be required to pay monetary remuneration to our Israeli employees for their inventions, even if the rights to such inventions have been duly assigned to us.
We enter into agreements with our Israeli employees pursuant to which such individuals agree that any inventions created in the scope of their employment are either owned exclusively by us or are assigned to us, depending on the jurisdiction, without the employee retaining any rights. A portion of our intellectual property has been developed by our Israeli employees during their employment for us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived by an employee during the course of his or her employment and within the scope of said employment are considered “service inventions. Service inventions belong to the employer by default, absent a specific agreement between the employee and employer otherwise. The Patent Law also provides that if there is no agreement regarding the remuneration for the service inventions, even if the ownership rights were assigned to the employer, the Israeli Compensation and Royalties Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for these inventions. The Committee has not yet determined the method for calculating this Committee-enforced remuneration. While it has previously been held that an employee may waive his or her rights to remuneration in writing, orally or by conduct, litigation is pending in the Israeli labor court is questioning whether such waiver under an employment agreement is enforceable. Although our Israeli employees have agreed that we exclusively own any rights related to their inventions, we may face claims demanding remuneration in consideration for employees’ service inventions. As a result, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could negatively affect our business.
We received Israeli government grants for certain of our research and development activities, the terms of which may require us to pay royalties and to satisfy specified conditions in order to manufacture products and transfer technologies outside of Israel. If we fail to satisfy these conditions, we may be required to pay penalties and refund grants previously received.
Our research and development efforts have been financed in part through royalty-bearing grants in an aggregate amount of approximately $2.46 million (including accumulated interest) that we received from the IIA as of December 31, 2020. With respect to the royalty-bearing grants we are committed to pay royalties at a rate of 3% to 3.5% on sales proceeds from our products that were developed under IIA programs up to the total amount of grants received, linked to the U.S. dollar and bearing interest at an annual rate of LIBOR applicable to U.S. dollar deposits. We are further required to comply with the requirements of the Israeli Encouragement of Industrial Research, Development and Technological Innovation Law, 5744-1984, as amended, and related regulations, or the Research Law, with respect to those past grants. When a company develops know-how, technology or products using IIA grants, the terms of these grants and the Research Law restrict the transfer or license of such know-how, and the transfer of manufacturing or manufacturing rights of such products, technologies or know-how outside of Israel, without the prior approval of the IIA. Therefore, the discretionary approval of an IIA committee would be required for any transfer or license to third parties inside or outside of Israel of know how or for the transfer outside of Israel of manufacturing or manufacturing rights related to those aspects of such technologies. We may not receive those approvals. Furthermore, the IIA may impose certain conditions on any arrangement under which it permits us to transfer technology or development.
The transfer or license of IIA-supported technology or know-how outside of Israel and the transfer of manufacturing of IIA-supported products, technology or know-how outside of Israel may involve the payment of significant amounts, depending upon the value of the transferred or licensed technology or know-how, our research and development expenses, the amount of IIA support, the time of completion of the IIA-supported research project and other factors. These restrictions and requirements for payment may impair our ability to sell, license or otherwise transfer our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction involving the transfer outside of Israel of technology or know-how developed with IIA funding (such as a merger or similar transaction) may be reduced by any amounts that we are required to pay to the IIA.
42
We may not be able to enforce covenants not-to-compete under current Israeli law that might result in added competition for our products.
We have non-competition agreements with all of our employees, all of which are governed by Israeli law. These agreements prohibit our employees from competing with or working for our competitors, generally during their employment and for up to 12 months after termination of their employment. However, Israeli courts are reluctant to enforce non-compete undertakings of former employees and tend, if at all, to enforce those provisions for relatively brief periods of time in restricted geographical areas, and only when the employee has obtained unique value to the employer specific to that employer’s business and not just regarding the professional development of the employee. If we are not able to enforce non-compete covenants, we may be faced with added competition.
Provisions of Israeli law and our amended and restated articles of association may delay, prevent or otherwise impede a merger with, or an acquisition of, us, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date on which a merger proposal is filed by each merging company with the Israel Registrar of Companies and at least 30 days have passed from the date on which the shareholders of both merging companies have approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, a tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless, following consummation of the tender offer, the acquirer would hold at least 98% of the Company’s outstanding shares. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, claim that the consideration for the acquisition of the shares does not reflect their fair market value, and petition an Israeli court to alter the consideration for the acquisition accordingly, unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek such appraisal rights, and the acquirer or the company published all required information with respect to the tender offer prior to the tender offer’s response date.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred. These provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders.
It may be difficult to enforce a judgment of a U.S. court against us and our executive officers and directors and the Israeli experts named in this prospectus in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our executive officers and directors and these experts.
We were incorporated in Israel. Substantially all of our executive officers and directors reside outside of the United States, and all of our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court (see “Enforceability of Civil Liabilities” for additional information on your ability to enforce a civil claim against us and our executive officers or directors named in this prospectus).
43
Your rights and responsibilities as a shareholder will be governed in key respects by Israeli laws, which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our Ordinary Shares are governed by our amended and restated articles of association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in U.S. companies. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain from abusing its power in such company, including, among other things, in voting at a general meeting of shareholders on matters such as amendments to a company’s amended and restated articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval, as well as a general duty to refrain from discriminating against other shareholders. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a vote at a meeting of the shareholders or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of these duties or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders of U.S. companies.
General Risk Factors
Our business may be impacted by changes in general economic conditions.
Our business is subject to risks arising from changes in domestic and global economic conditions, including adverse economic conditions in markets in which we operate, which may harm our business. For example, the current COVID-19 pandemic has caused significant volatility and uncertainty in U.S. and international markets. If our future customers significantly reduce spending in areas in which our technology and products are utilized, or prioritize other expenditures over our technology and products, our business, financial condition, results of operations and prospects would be materially adversely affected.
Disruption to the global economy could also result in a number of follow-on effects on our business, including a possible slow-down resulting from lower customer expenditures; inability of customers to pay for products, solutions or services on time, if at all; more restrictive export regulations which could limit our potential customer base; negative impact on our liquidity, financial condition and share price, which may impact our ability to raise capital in the market, obtain financing and secure other sources of funding in the future on terms favorable to us.
In addition, the occurrence of catastrophic events, such as hurricanes, storms, earthquakes, tsunamis, floods, medical epidemics and other catastrophes that adversely affect the business climate in any of our markets could have a material adverse effect on our business, financial condition and results of operations. Some of our operations are located in areas that have been in the past, and may be in the future, susceptible to such occurrences.
Changes in financial accounting standards may cause adverse and unexpected revenues fluctuations and impact our results of operations.
A change in accounting standards or practices could harm our operating results. New accounting pronouncements and varying interpretations of accounting pronouncements have occurred and may occur in the future. Changes to existing rules or the questioning of current practices may harm our operating results or the way we conduct our business.
The JOBS Act will allow us to postpone the date by which we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the SEC, which could undermine investor confidence in our company and adversely affect the market price of the Ordinary Shares.
For so long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies” including:
| ● | the provisions of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
44
| ● | Section 107 of the JOBS Act, which provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are electing to delay the adoption of some of the new or revised accounting standards. As a result of this adoption, our financial statements may not be comparable to companies that comply with the public company effective date; | |
| ● | any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements; and | |
| ● | our ability to furnish two rather than three years of income statements and statements of cash flows in various required filings. |
We intend to take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the date of our first sale of common equity securities pursuant to an effective registration statement under the Securities Act, (b) in which we have total annual gross revenues of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Ordinary Shares that is held by non-affiliates exceeds $700 million as of the prior June 30, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
We cannot predict if investors will find the Ordinary Shares less attractive because we may rely on these exemptions. If some investors find the Ordinary Shares less attractive as a result, there may be a less active trading market for the Ordinary Shares, and our market prices may be more volatile and may decline.
As a “foreign private issuer” we are subject to less stringent disclosure requirements than domestic registrants and are permitted, and may in the future elect to follow certain home country corporate governance practices instead of otherwise applicable SEC and Nasdaq requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. registrants.
As a foreign private issuer and emerging growth company, we may be subject to different disclosure and other requirements than domestic U.S. registrants and non-emerging growth companies. For example, as a foreign private issuer, in the United States, we are not subject to the same disclosure requirements as a domestic U.S. registrant under the Exchange Act, including the requirements to prepare and issue quarterly reports on Form 10-Q or to file current reports on Form 8-K upon the occurrence of specified significant events, the proxy rules applicable to domestic U.S. registrants under Section 14 of the Exchange Act or the insider reporting and short-swing profit rules applicable to domestic U.S. registrants under Section 16 of the Exchange Act. In addition, we intend to rely on exemptions from certain U.S. rules which will permit us to follow Israeli legal requirements rather than certain of the requirements that are applicable to U.S. domestic registrants.
We will follow Israeli laws and regulations that are applicable to Israeli companies. However, Israeli laws and regulations applicable to Israeli companies do not contain any provisions comparable to the U.S. proxy rules, the U.S. rules relating to the filing of reports on Form 10-Q or 8-K or the U.S. rules relating to liability for insiders who profit from trades made in a short period of time, as referred to above.
Furthermore, foreign private issuers are required to file their annual report on Form 20-F within 120 days after the end of each fiscal year, while U.S. domestic registrants that are non-accelerated filers are required to file their annual report on Form 10-K within 90 days after the end of each fiscal year. Foreign private issuers are also exempt from Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information, although we will be subject to Israeli laws and regulations having substantially the same effect as Regulation Fair Disclosure. As a result of the above, even though we are required to file reports on Form 6-K disclosing the limited information which we have made or are required to make public pursuant to Israeli law, or are required to distribute to shareholders generally, and that is material to us, you may not receive information of the same type or amount that is required to be disclosed to shareholders of a U.S. registrant.
These exemptions and leniencies will reduce the frequency and scope of information and protections to which you are entitled as an investor.
45
The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, the next determination will be made with respect to us on June 30, 2021. In the future, we would lose our foreign private issuer status if a majority of our shareholders, directors or management are U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic registrant may be significantly higher.
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
We will incur significant increased costs as a result of the listing of our securities for trading on Nasdaq. By becoming a public company in the United States, our management will be required to devote substantial time to new compliance initiatives as well as compliance with ongoing U.S. requirements.
Upon the listing of securities on Nasdaq, we will become a publicly traded company in the United States. As a public company in the United States, we will incur additional significant accounting, legal and other expenses that we did not incur before the offering. We also anticipate that we will incur costs associated with corporate governance requirements of the SEC, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. We expect these rules and regulations to increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and to make some activities more time consuming and costly. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs. Any future changes in the laws and regulations affecting public companies in the United States, including Section 404 and other provisions of the Sarbanes-Oxley Act, and the rules and regulations adopted by the SEC, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees, or as executive officers.
Our business and operations might be adversely affected by security breaches, including any cybersecurity incidents.
We depend on the efficient and uninterrupted operation of our computer and communications systems, and those of our consultants, contractors and vendors, which we use for, among other things, sensitive company data, including our intellectual property, financial data and other proprietary business information.
While certain of our operations have business continuity and disaster recovery plans and other security measures intended to prevent and minimize the impact of IT-related interruptions, our IT infrastructure and the IT infrastructure of our consultants, contractors and vendors are vulnerable to damage from cyberattacks, computer viruses, unauthorized access, electrical failures and natural disasters or other catastrophic events. We could experience failures in our information systems and computer servers, which could result in an interruption of our normal business operations and require substantial expenditure of financial and administrative resources to remedy. System failures, accidents or security breaches can cause interruptions in our operations and can result in a material disruption of our targeted phage therapies, product candidates and other business operations. The loss of data from completed or future studies or clinical trials could result in delays in our research, development or regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur regulatory investigations and redresses, penalties and liabilities and the development of our product candidates could be delayed or otherwise adversely affected.
Even though we believe we carry commercially reasonable business interruption and liability insurance, we might suffer losses as a result of business interruptions that exceed the coverage available under our insurance policies or for which we do not have coverage. For example, we are not insured against terrorist attacks or cyberattacks. Any natural disaster or catastrophic event could have a significant negative impact on our operations and financial results. Moreover, any such event could delay the development of our product candidates.
46
Our business and operations would suffer in the event of computer system failures, cyber-attacks or a deficiency in our cybersecurity.
Despite the implementation of security measures intended to secure our data against impermissible access and to preserve the integrity and confidentiality of our data, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from computer viruses, malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our new products development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur material legal claims and liability, including under data privacy laws such as the GDPR, damage to our reputation, and the further development of our new products could be delayed.
Sales of a significant number of shares of our Ordinary Shares in the public markets or significant short sales of our Ordinary Shares, or the perception that such sales could occur, could depress the market price of our Ordinary Shares and impair our ability to raise capital.
Sales of a substantial number of shares of our Ordinary Shares or other equity-related securities in the public markets, could depress the market price of our Ordinary Shares. If there are significant short sales of our Ordinary Shares, the price decline that could result from this activity may cause the share price to decline more so, which, in turn, may cause long holders of the Ordinary Shares to sell their shares, thereby contributing to sales of Ordinary Shares in the market. Such sales also may impair our ability to raise capital through the sale of additional equity securities in the future at a time and price that our management deems acceptable, if at all.
The market price of our Ordinary Shares may be highly volatile, and you could lose all or part of your investment.
The market price of our Ordinary Shares is likely to be volatile. This volatility may prevent you from being able to sell your Ordinary Shares at or above the price you paid for your securities. Our share price could be subject to wide fluctuations in response to a variety of factors, which include:
| ● | whether we achieve our anticipated corporate objectives; | |
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; | |
| ● | changes in our financial or operational estimates or projections; | |
| ● | our ability to implement our operational plans; | |
| ● | termination of the lock-up agreement or other restrictions on the ability of our stockholders to sell shares after this offering; | |
| ● | changes in the economic performance or market valuations of companies similar to ours; and | |
| ● | general economic or political conditions in the United States or elsewhere. |
In addition, the stock market in general, and the stock of publicly-traded medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our Ordinary Shares, regardless of our actual operating performance, and we have little or no control over these factors.
47
Our securities will be traded on more than one market or exchange and this may result in price variations.
Our Ordinary Shares have been trading on the TASE since February 2, 2011. Assuming that our Ordinary Shares are listed for trading on the Nasdaq, trading in our Ordinary Shares will take place in different currencies (U.S. dollars on the Nasdaq and NIS on the TASE), and at different times (resulting from different time zones, trading days, and public holidays in the United States and Israel). The trading prices of our securities on these two markets may differ due to these and other factors. Any decrease in the price of our Ordinary Shares on the TASE could cause a decrease in the trading price of our Ordinary Shares on the Nasdaq.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or the Ordinary Shares, our share price and trading volume could decline.
The trading market for the Ordinary Shares will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding the Ordinary Shares, or provide more favorable relative recommendations about our competitors, the price of our Ordinary Shares would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our Ordinary Shares or trading volume to decline.
48
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere in this prospectus constitute forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” “intends” or “continue,” or the negative of these terms or other comparable terminology.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, expected capital needs and expenses, statements relating to the research, development, completion and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate
Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
| ● | our planned level of revenues and capital expenditures; | |
| ● | our ability to market and sell our products; | |
| ● | our plans to continue to invest in research and development to develop technology for both existing and new products; | |
| ● | our ability to maintain our relationships with suppliers, manufacturers and other partners; | |
| ● | our ability to maintain or protect the validity of our European, U.S. and other patents and other intellectual property; | |
| ● | our ability to retain key executive members; | |
| ● | our ability to internally develop and protect new inventions and intellectual property; | |
| ● | our ability to expose and educate physicians and other medical professionals about the use cases of our products; | |
| ● | our expectations regarding our tax classifications; | |
| ● | interpretations of current laws and the passages of future laws; and | |
| ● | the impact of COVID-19 and resulting government actions on us, our manufacturers, suppliers and facilities in which our ProSense system is used or in which our products are undergoing trials. |
These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading “Risk Factors” and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus.
49
Our Ordinary Shares have been trading on the TASE under the symbol “ICCM” since February 2011. We have applied to list the Ordinary Shares on Nasdaq under the symbol “ICCM.” Although we believe that as of the closing of the January 2021 SPA we meet the initial listing criteria for listing our Ordinary Shares on Nasdaq, there can be no assurance that our application will be approved. As of the date of this prospectus our only listed class of securities will be Ordinary Shares outstanding. All of the Ordinary Shares, including those to be offered by the selling shareholders pursuant to this prospectus, have the same rights and privileges see “Description of Share Capital And Governing Documents”).
We will not receive any proceeds from the sale of the Ordinary Shares by the selling shareholders. All net proceeds from the sale of the Ordinary Shares will go to the selling shareholders.
We have never declared or paid any cash dividends on our Ordinary Shares and do not anticipate paying any cash dividends in the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our board of directors and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
The Companies Law imposes further restrictions on our ability to declare and pay dividends.
Payment of dividends may be subject to Israeli withholding taxes (see “Taxation” for additional information).
50
The following table sets forth our cash and cash equivalents and our capitalization as of December 31, 2020:
| ● | on an actual basis; and | |
| ● | on an pro forma basis to give additional effect to the issuance of 91,885,555 Ordinary Shares and cash proceed of $15 million pursuant to the January 2021 SPA. |
You should read this table in conjunction with the sections titled “Summary Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
As of December 31, | ||||||||
| U.S. dollars in thousands | Actual | Pro Forma | ||||||
| Cash and cash equivalents | 3,502 | 18,099 | ||||||
| Deposit | 4,669 | 4,669 | ||||||
| Shareholders’ equity: | ||||||||
| Ordinary shares | 4,524 | 4,524 | ||||||
| Treasury shares | (41 | ) | (41 | ) | ||||
| Additional paid-in capital | 49,701 | 64,298 | ||||||
| Accumulated deficit | (48,536 | ) | (48,536 | ) | ||||
| Total shareholders’ equity | 5,648 | 20,245 | ||||||
| Total capitalization | 6,525 | 21,122 | ||||||
51
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified in “Cautionary Note Regarding Forward-Looking Statements” and under “Risk Factors” elsewhere in this prospectus.
Overview
We are a commercial stage medical device company focusing on the research, development and marketing of cryoablation systems and technologies based on LN2 for treating tumors. Cryoablation is the process by which benign and malignant tumors are ablated (destroyed) through freezing such tumors while in a patient’s body. Our proprietary cryoablation technology is a minimally invasive alternative to surgical intervention, for tumors, including those found in breast, lungs, kidneys and bones and other indications. Our lead commercial cryoablation product is the ProSense system.
In addition to our existing lead product, the ProSense system, a single probe system, we have developed an additional multi probe system that is expected to have the ability to freeze several tumors simultaneously or larger tumors, which we refer to as our MultiSense system. Currently, we are focusing on developing our next generation MultiSense system, which we intend to commercialize subject to regulatory approvals. In our continued efforts aimed at improving our core technology, we are also currently in the process of developing our next generation single probe system. While these next generation systems are still in various research and development stages, we expect them to be more efficient and user friendly (see “Business – Our Products – Research and Development” for additional information).
Components of Operating Results
Revenues
Our revenues primarily consist of (i) selling our ProSense system and its disposables and related services; and (ii) revenues from granting the exclusive distribution rights to our products in Japan and Singapore to Terumo Corporation, which also include providing technical, regulatory and clinical materials and support in obtaining regulatory approvals.
Cost of Revenues
Our cost of revenues consists primarily of salaries and related personnel expenses, materials for production of our products, subcontractors’ expenses and other related production expenses.
Gross Margin
Gross margin, or gross profit as a percentage of revenue, is affected by a variety of factors which influence our revenues and the cost of goods sold. Revenues are affected mostly by the different selling prices depending on sales channels, territories and the mix of products and currency fluctuation, mainly the U.S. Dollar against the EURO. The cost of revenues is affected mostly by the changes in cost of materials and import costs, subcontractors’ costs, cost of personal, and currency fluctuation, mainly the U.S. Dollar against the NIS. Our gross margin, is also affected by production volumes and production efficiency.
Operating Expenses
Our current operating expenses consist of three components — research and development expenses, marketing and sales expenses and general and administrative expenses.
Research and Development Expenses
Our research and development expenses consist primarily of salaries and related benefits, subcontractor’s expenses, materials and other related research and development expenses, clinical studies and regulation expenses.
We expect that our research and development expenses will materially increase as we continue to develop our new products, pursue new regulatory indications in the US and other territories, collect updated clinical data, and recruit additional research and development and regulation employees.
52
Sales and Marketing
Our sales and marketing expenses consist primarily of salaries and related benefits, payments to consultants, costs associated with conventions, travel and other marketing and sales expenses.
We expect that our sales and marketing expenses will materially increase as we continue to enhance our market penetration efforts and recruit additional sales and marketing employees.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits, professional services fees for accounting, legal, directors’ fees, facilities, and associate costs, insurance and other general and administrative expenses. We expect our general and administrative expenses to increase as a result of the listing on Nasdaq and expansion of our business.
Financial expense and income
Financial expenses and income consist primarily of exchange rate differences, interest expenses on cash and cash equivalents, deposits and other assets and liabilities which are denominated in NIS and Euros.
Comparison of the Years Ended December 31, 2020 and 2019
Results of Operations
The following table summarizes our results of operations for the periods presented.
Year Ended December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Revenues | 3,868 | 1,627 | ||||||
| Cost of revenues | 1,424 | 1,103 | ||||||
| Gross profit | 2,444 | 524 | ||||||
| Research and development expenses | 3,809 | 3,001 | ||||||
| Marketing and sales expenses | 1,063 | 1,035 | ||||||
| General and administrative expenses | 1,714 | 1,272 | ||||||
| Operating loss | 4,142 | 4,784 | ||||||
| Financial income, net | (412) | (233 | ) | |||||
| Net loss and comprehensive loss | 3,730 | 4,551 | ||||||
| Basic and diluted net loss per share | 0.027 | 0.042 | ||||||
Revenues
The following table summarizes our revenues through types for the periods presented. The period-to-period comparison of results is not necessarily indicative of results for future periods.
Year Ended December 31, | ||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Systems | 1,817 | 786 | ||||||
| Disposables | 1,006 | 493 | ||||||
| Exclusive distribution agreement | 1,045 | 348 | ||||||
| Total | 3,868 | 1,627 | ||||||
53
The following table summarizes our revenues by geographic region for the periods presented. The period-to-period comparison of results is not necessarily indicative of results for future periods.
Year Ended December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Japan | 2,097 | 512 | ||||||
| United States | 276 | 231 | ||||||
| China | - | 220 | ||||||
| Spain | 207 | 211 | ||||||
| Israel | 7 | 65 | ||||||
| Thailand | 528 | - | ||||||
| Other | 753 | 388 | ||||||
| Total | 3,868 | 1,627 | ||||||
Our revenues for the year ended December 31, 2020 increased by 138% to $3,868 thousand, compared to $1,627 thousand revenues for the year ended December 31, 2019. The increase is attributable to an increase of 131% in revenues from sales of our systems which amounted to revenues of $1,817 thousand for the year ended December 31, 2020 compared to $786 thousand for the year ended December 31, 2019, increase of 104% in revenues from sales of our probes to $1,006 thousand for the year ended December 31, 2020 compared to $493 thousand for the year ended December 31, 2019, and increase of 200% in revenues from our exclusive distribution agreement with Terumo Corporation to $1,045 thousand for the year ended December 31, 2020 compared to $348 thousand for the year ended December 31, 2019.
Accordingly, our revenues in Japan increased by 310% to $2,097 thousand for the year ended December 31, 2020, of which $1,052 are from sales of products, most of which generated from Terumo Corporation’s initial order, and $1,045 are revenues from our exclusive distribution agreement with Terumo Corporation. For the year ended December 31, 2019, our revenues from sales of products in Japan were $164 thousand and our revenues from our exclusive distribution agreement with Terumo Corporation were $348 thousand. Our revenues from sales in the United States increased by 19% to $276 thousand for the year ended December 31, 2020, compared to $231 thousand for the year ended December 31, 2019. We did not have any sales in China in 2020 since we are in the process to obtain regulatory approvals for our Probes. In Thailand, following obtaining regulatory approval, our revenues in 2020 were $528 thousand compared to no sales in 2019.
Cost of Revenues and Gross Profit
The following table summarizes our cost of revenues for the periods presented, as well as presenting the gross profit as a percentage of total revenues. The period-to-period comparison of results is not necessarily indicative of results for future periods.
Year Ended December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Payroll and related benefits (including Share-based compensation) | 655 | 473 | ||||||
| Raw materials, subcontractors, and auxiliary materials (including changes in inventories) | 461 | 389 | ||||||
| Shipping | 39 | 69 | ||||||
| Royalties to IIA | 116 | 48 | ||||||
| Others | 153 | 124 | ||||||
| Total | 1,424 | 1,103 | ||||||
| Gross profit | 2,444 | 524 | ||||||
| Gross profit % | 63.1 | % | 32.2 | % | ||||
Our cost of revenues for the year ended December 31, 2020 increased by 29.1% to $1,424 thousand, compared to $1,103 thousand for the year ended December 31, 2019, whereas our gross profit for the year ended December 31, 2020 increased by 366% to $2,444 thousand, which is 63.2% of our revenues in 2020, compared to $1,103 thousand for the year ended December 31, 2019 which is 32.2% of our revenues in 2019. The increase in absolute gross profit is primarily due to increase in volume of sales of products and revenues from our exclusive distribution agreement with Terumo Corporation. The increase in our gross profit margin is primarily attributable to: (i) allocation of fixed costs and overhead expenses on a higher volume of sales; (ii) increased efficiency production process; and (iii) revenues from our exclusive distribution agreement with Terumo Corporation which did not have corresponding production expenses.
54
Research and development expenses
The following table summarizes our research and development costs for the periods presented. The period-to-period comparison of results is not necessarily indicative of results for future periods.
Year Ended December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Payroll and related benefits (including share-based compensation) | 2,273 | 1,730 | ||||||
| Raw materials, subcontracted work and consulting | 1,058 | 866 | ||||||
| Others | 478 | 405 | ||||||
| Total | 3,809 | 3,001 | ||||||
Research and development, or R&D, expenses increased by 26.9% to $3,809 thousand 2020, compared with $3,001 thousand in 2019. This increase reflects our strategy regarding focusing on the development of our next generation MultiSense, and single probe systems, and regulatory activity. The increase is primarily due to increase in employee related costs, raw materials subcontractors and consulting.
Sales and marketing expenses
The following table summarizes our sales and marketing costs for the periods presented. The period-to-period comparison of results is not necessarily indicative of results for future periods.
Year Ended December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Payroll and related benefits (including Share-based compensation) | 576 | 403 | ||||||
| Consultants and professional services | 157 | 150 | ||||||
| Travel | 29 | 167 | ||||||
| Advertising and promotion expenses | 36 | 41 | ||||||
| Sales Commissions | 57 | 33 | ||||||
| Others | 208 | 241 | ||||||
| Total | 1,063 | 1,035 | ||||||
Selling and marketing expenses for the year ended December 31, 2020 increased by 2.7% to $1,063 thousand, compared to $1,035 thousand in 2019. The increase of selling and marketing expenses compared in 2020, reflects our strategy regarding the expansion of our marketing activities, that was limited in part due to restrictions imposed due to COVID-19. The increase during the year 2020 compared to the year 2019 is mostly due to an increase in salary and related expenses and sales commissions, which was partially offset by a decrease in travel expenses following the spread of COVID-19, and other selling and marketing expenses.
General and administrative expenses
The following table summarizes our general and administrative costs for the periods presented. The period-to-period comparison of results is not necessarily indicative of results for future periods.
| Year
Ended December 31, | ||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Payroll and related benefits (including Share-based compensation) | 965 | 833 | ||||||
| Professional services | 611 | 347 | ||||||
| Others | 138 | 92 | ||||||
| Total | 1,714 | 1,272 | ||||||
General and administrative expenses increased by 34.7% to $1,714 thousand for the year ended December 31, 2020, compared to $1,272 thousand for the year ended December 31, 2019. This increase is primarily due to additional payroll and related benefits, legal, audit and other expenses relating to the preparation of the listing of the Company’s Ordinary Shares on Nasdaq.
55
Operating loss
Based on the foregoing, our operating loss decreased from $4,784 thousand for the year ended December 31, 2019 to $4,142 thousand for the year ended December 31, 2020.
Financial income, net
Financial income, net for the year ended December 31, 2020 was $412 thousand compared to $233 thousand for the year ended December 31, 2019. The increase in our financial income net is primarily because of the effect of the NIS strengthening against the U.S. dollar on our net NIS denominated assets, including cash and cash equivalents, deposits and other net liquid liabilities.
Net loss
Net loss for the year ended December 31, 2020 decreased by $821 thousand or 18%, to $3,730 thousand, compared with a net loss of $4,551 thousand for the year ended December 31, 2019. This decrease is primarily attributable to: (i) the increase in revenues and gross profit which attributed to the decrease in operating loss, and (ii) the increase in net financial income, as described above.
Critical Accounting Policies and Estimates
We describe our significant accounting policies more fully in Note 2 to our financial statements for the year ended December 31, 2019. We believe that the accounting policy below is critical in order to fully understand and evaluate our financial condition and results of operations.
We prepare our financial statements in accordance with U.S. GAAP. At the time of the preparation of the financial statements, our management is required to use estimates, evaluations, and assumptions which affect the application of the accounting policy and the amounts reported for assets, obligations, income, and expenses. Any estimates and assumptions are continually reviewed. The changes to the accounting estimates are credited during the period in which the change to the estimate is made.
Use of estimates in the preparation of financial statements:
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made. Actual results could differ from those estimates.
Share-based compensation
We measure the compensation costs of share-based compensation arrangements based on the grant-date fair value and recognize the costs in the financial statements over the period during which employees are required to provide services. Share-based compensation arrangements include options, performance-based awards, share appreciation rights, and employee share purchase plans. We amortize such compensation amounts, if any, over the respective service periods of the award. We use the Black-Scholes-Merton option pricing model, or the Black-Scholes Model, an acceptable model in accordance with ASC 718, Compensation-Stock Compensation, to value options. Option valuation models require the input of assumptions, including the expected life of the stock-based awards, the estimated stock price volatility, the risk-free interest rate, and the expected dividend yield. The risk-free interest rate assumption is based upon the yield from Israel Treasury zero-coupon bonds with an equivalent term. Estimated volatility is a measure of the amount by which our stock price is expected to fluctuate each year during the term of the award. Our calculation of estimated volatility is based on historical stock prices over a period equal to the expected term of the awards. The average expected life of options was based on the contractual terms of the stock option using the simplified method. We utilize a dividend yield of zero based on the fact that we have never paid cash dividends and have no current intention to pay cash dividends. The assumptions used in calculating the fair value of share-based awards represent our best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our share-based compensation expense could be materially different in the future. We recognize the compensation expense for share-based compensation granted based on the grant date fair value estimated in accordance with ASC 718. We generally recognize the compensation expense over the employee’s requisite service period. We account for forfeitures when they occur.
56
Liquidity and Capital Resources
Overview
Since our inception through December 31, 2020, we have funded our operations principally from the issuance of Ordinary Shares, options, convertible securities, loans, revenues from sale of products and grants received from the IIA. As of December 31, 2020, we had approximately $ 8,171 thousand in cash and cash equivalents and short-term bank deposits, compared with $5,789 thousand as of December 31, 2019.
The table below presents our cash flows for the periods indicated.
| Year
Ended December 31, |
||||||||
| U.S. dollars in thousands | 2020 | 2019 | ||||||
| Net cash used in operating activities | (3,690 | ) | (1,989 | ) | ||||
| Net cash used in investing activities | (4,670 | ) | (103 | ) | ||||
| Net cash provided by financing activities | 5,858 | 3,309 | ||||||
| Effect of foreign currency exchange rates on cash and cash equivalents: | 215 | 358 | ||||||
| Net increase (decrease) in cash and cash equivalents | (2,287) | 1,575 | ||||||
Operating Activities
Cash flows from operating activities consist primarily of loss adjusted for various non-cash items, including depreciation and amortization and share-based compensation expenses. In addition, cash flows from operating activities are impacted by changes in operating assets and liabilities, which include inventories, accounts receivable, other assets, accounts payable and other current liabilities.
Net cash used in operating activities for the year ended December 31, 2020 was $3,690 thousand. This net cash used in operating activities primarily reflects a net loss of $3,730 thousand which is decreased by net non-cash expenses of $77 thousand and increase net of $37 thousand which reflects changes in operating assets and liabilities.
The decrease in operating assets and liabilities for the year ended December 31, 2020 is attributed mainly to an increase in net cash loss of $37 thousand in trade accounts receivable, deposit, inventory, trade accounts payable and other long-term liabilities.. This increase was partially offset due to decrease in trade accounts payable and other long-term liabilities.
Net cash used in operating activities for the year ended December 31, 2019 was $1,989 thousand. This net cash used in operating activities primarily reflects a net loss of $4,551 thousand which is decreased by net non-cash expenses of $46 thousand and decrease net of $2,516 thousand which reflects changes in operating assets and liabilities.
The increase in operating assets and liabilities for the year ended December 31, 2019 is attributed mainly to a decrease in cash loss of $2,306 thousand in other current liabilities due to advance payments received mostly from Terumo Corporation under the exclusive distribution agreement. This decrease was partially offset due to increase in prepaid expenses and other receivables and increase in right of use assets.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2020 was $4,670 thousand. This net cash used in investing activities is primarily attributable to investment in short-term bank deposits in the amount of $4,432 thousand and purchase of property and equipment of $223 thousand. Net cash used in investing activities for the year ended December 31, 2019 was $103 thousand. This cash used in investing activities is attributable to purchase of property and equipment in an aggregate total amount of $103 thousand.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2020 was $5,858 thousand. This net cash is attributed primarily to net proceeds from issuance of Ordinary Shares in a public offering in the net amount of $5,847 and exercise of options in the amount of $11 thousand.
Net cash provided by financing activities for the year ended December 31, 2019 was $3,309 thousand which is attributed to net proceeds from issuance of shares in a public offering and a rights offering.
57
Financial Arrangements
As of July 6, 2021, our credit arrangements include grants from the IIA.
Since our inception through our initial public offering on TASE on February 2, 2011, we have funded our operations primarily through the private sale of equity securities and convertible debt in an aggregate amount of $8.9 million.
Since our initial public offering on TASE through March 2018, we raised an aggregate of $29.7 million, in public offerings of equity securities, rights offerings and convertible debt, and a private placement to Epoch Investment Partners Limited, or the Epoch SPA, who became our controlling shareholder on February 7, 2015. Pursuant to the Epoch SPA, we received a total of $5,485 thousand against issuance of 15,142,857 Ordinary Shares and 2,271,429 warrants. The warrants were not exercised and expired. Since than we performed multiple public offerings and rights offerings.
Since March 2018, we have funded our operations mainly through a number of public and rights offerings in an aggregate amount of $11.6 million.
On August 12, 2020, we received an unsecured loan under the United States Small Business Administration, or the SBA, PPP pursuant to the Coronavirus Aid, Relief, and Economic Security Act in the amount of $24,898. The five-year SBA-administered PPP loan has an interest rate of 1.0% per annum. According to the terms of the loan, as long as a borrower submits its loan forgiveness application within10 months of the completion of the covered period of such loan, the borrower is not required to make any payments until the forgiveness amount is remitted to the lender by SBA. If the loan is fully forgiven, the borrower is not responsible for any payments. If only a portion of the loan is forgiven, or if the forgiveness application is denied, any remaining balance due on the loan must be repaid by the borrower on or before the maturity date of the loan. Interest accrues during the time between the disbursement of the loan and SBA remittance of the forgiveness amount. In April 2021, we submitted a loan forgiveness application.
On January 26, 2021, we entered into the January 2021 SPA with the January 2021 Investors. Pursuant to the January 2021 SPA, we will receive an aggregate amount of $15 million, against issuance of 91,885,555 Ordinary Shares. The January 2021 Investors were granted a 12-month participation right following the January 2021 Second Closing, in future financings equal to 50% of the subsequent financing, subject to certain conditions. We also undertook to refrain from issuing any Ordinary Shares or Ordinary Shares equivalents from the date of the January 2021 SPA until 60 calendar days from the January 2021 Second Closing, subject to certain exempt issuances. On March 9, 2021, we received $9 million and issued 55,131,333 Ordinary Shares, and on May 9, 2021, we received $6 million and issued 36,754,222 Ordinary Shares.
In addition, since our inception, we received an aggregate of $2.46 million (including accumulated interest) from the IIA.
Current Outlook
We have financed our operations to date primarily through proceeds from sales of our Ordinary Shares and convertible securities, sales of our products and grants from the IIA. We have incurred losses and generated negative cash flows from operations since inception in 2006. Since 2012, we have generated revenues from the sale of our products and revenues from granting the exclusive distribution rights to our products in Japan and Singapore to Terumo Corporation, which also include providing technical, regulatory and clinical materials and support in obtaining regulatory approvals.
We expect to generate revenues from the sale of our products and other revenues in the future. However, we do not expect these revenues to support all of our operation in the near future. We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the development of our MultiSense system, and continue our commercialization efforts. Furthermore, following the completion of this listing, we expect to incur additional costs associated with operating as a Nasdaq public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations.
As of December 31, 2020, our cash, cash equivalents and short-term deposits were $8,171 thousand, and we had a working capital of $5,875 thousand and an accumulated deficit of $48,536 thousand. On March 9, 2021, pursuant to the January 2021 SPA, we received $9,000 thousand. On May 9, 2021, pursuant to the January 2021 SPA, we received $6,000 thousand. As of June 30, 2021, our cash, cash equivalents and short-term deposits were $18,607 thousand. We expect that our existing cash, cash equivalents and short-term deposits will be sufficient for the next 12 months of operation; however, we expect that we will require substantial additional capital to continue the development of, and to commercialize our products. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future capital requirements will depend on many factors, including:
58
| ● | our ability to sell our products according to our plans; | |
| ● | the progress and cost of our research and development activities; | |
| ● | the costs associated with the manufacturing our products; |
| ● | the costs of our clinical trials and obtaining regulatory approvals; | |
| ● | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; | |
| ● | the cost of our commercialization efforts, marketing, sales and distribution of our products the potential costs of contracting with third parties to provide marketing and distribution services for us or for building such capacities internally; and | |
| ● | the magnitude of our general and administrative expenses. |
Until we can generate significant recurring revenues and profit, we expect to satisfy our future cash needs through debt or equity financings. We cannot be certain that additional funding will be available to us when needed, on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans, and/or commercialization efforts and/or regulatory efforts with respect to our products in different territories. This may raise substantial doubts about our ability to continue as a going concern.
Off-Balance Sheet Arrangements
We do not have any relationships with unconsolidated entities or financial partnerships, including entities sometimes referred to as structured finance or special purpose entities that were established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. We do not engage in off-balance sheet financing arrangements. In addition, we do not engage in trading activities involving non-exchange traded contracts.
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2020.
| Total | Less
than 1 year | 1-3 years | 3-5 years | More
than 5 years | ||||||||||||||||
| Operating leases | $ | 369 | $ | 218 | $ | 151 | - | - | ||||||||||||
Operating lease obligations consist of payments pursuant to lease agreements for our facility in Israel and motor vehicle leases.
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our current investment policy is to invest available cash in bank deposits with banks that have a credit rating of at least A-minus. Accordingly, some of our cash and cash equivalents is held in deposits that bear interest. Given the current low rates of interest we receive, we will not be adversely affected if such rates are reduced. Our market risk exposure is primarily a result of U.S. dollar/NIS exchange rates, which is discussed in detail in the following paragraph.
Foreign Exchange Risk
Our results of operations and cash flow are subject to fluctuations due to changes in U.S. dollar/NIS currency exchange rates. A certain portion of our liquid assets is held in U.S. dollars, and the vast majority of our expenses is denominated in NIS. Changes of 5% and 10% in the U.S. dollar/NIS exchange rate would increase/decrease our operating expenses for December 31, 2020 by approximately 8% and 4%, respectively. However, these historical figures may not be indicative of future exposure. Currently, we do not hedge our foreign currency exchange risk. In the future, we may enter into formal currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
59
Overview
We are a commercial stage medical device company focusing on the research, development and marketing of cryoablation systems and technologies based on liquid nitrogen, or LN2, for treating tumors. Cryoablation is the process by which benign and malignant tumors are ablated (destroyed) through freezing such tumors while in a patient’s body. Our proprietary cryoablation technology is a minimally invasive alternative to surgical intervention, for tumors, including those found in breast, lungs, kidneys, bones and other indications. Our lead commercial cryoablation product is the ProSense system (pictured below).

In addition to our existing lead product, the ProSense system, a single probe system, we have developed an additional multi probe system that is expected to have the ability to freeze several tumors simultaneously or larger tumors, which we refer to as our MultiSense system. In our continued efforts aimed at improving our core technology, we are currently focusing on developing our next generation MultiSense system, which we intend to commercialize subject to regulatory approvals. We are also currently in the process of developing our next generation single probe system. While these next generation systems are still in various research and development stages, we expect them to be more efficient and user friendly (see “Business – Our Products – Research and Development” for additional information).
We believe that obtaining regulatory approval for our products for specific indications will help us grow our business. As of July 2021, we have received a broad range of regulatory approvals for our systems to treat tumors in the lungs, kidneys, bones and other indications. In the United States our products are approved as a “single family” known as the “IceCure Family,” which includes the IceSense3, ProSense, and MultiSense (which has not been commercialized) cryoablation systems. Although our existing, “IceCure Family” systems have regulatory approvals from the FDA for commercialization in the United States, we have yet to receive regulatory approval for such systems for treatment of malignant breast tumors, which requires a separate approval from the FDA. The FDA classifies medical devices into one of three classes (Class I, Class II, or Class III) depending on their level of risk and the types of controls that are necessary to ensure device safety and effectiveness. The class assignment is a factor in determining the type of premarketing submission or application, if any, that will be required before marketing products in the United States. If the FDA does not approve 510(k) regulatory pathway, we will request that De Novo classification be accepted for our “IceCure Family” systems. If De Novo classification is needed for our “IceCure Family” systems, we will be required to accept special controls imposed by the FDA, mainly on the production process and post-market monitoring. If a De Novo classification is not approved by the FDA as the regulatory pathway, the FDA will accept only PMA, in which case we expect the timeline for marketing approvals would be longer and our costs associated with PMA would be higher compared to 510(k) or De Novo approvals (see “Business – Government Regulation” for additional information).
60
Obtaining regulatory approvals and developing our next generation MultSense system will require the incurrence of significant costs and our success will depend, in part, on gaining market acceptance. In order to gain market acceptance in the United States, we will require specific approval from the FDA for our ProSense system, which we hope to obtain based on the interim results of our ICE3 trial published on April 29, 2021 , obtaining Medicare coverage from the Medicare Coverage of Innovative Technology, or MCIT, the American Society of Breast Surgeons, or the ASBrS, amending their guidelines to support cryoablation as an alternative to surgery, applying for CPT1 codes for cryoablation for breast cancer (with the support of the ASBrS), negotiate medical coverage for our systems from medical insurers and collaborating with a major distributor of medical devices (see “Business – Government Regulation – FDA Regulation of Medical Devices” and “Risk Factors – Risks Related to Product Development and Regulatory Approval” for additional information).
In addition, competition in the medical devices and cancer treatment market is intense. Some of our competitors hold significant market share, have long histories and strong reputations within the industry, greater brand recognition, financial and human resources than we do. They also have more experience and capabilities in researching and developing testing devices, obtaining and maintaining regulatory clearances and other requirements, manufacturing and marketing those products than we do. Their dominant market position and significant control over the market could significantly limit our ability to introduce or effectively market and generate sales of our ProSense and MultiSense systems.
To date, we have incurred significant operating losses, generated minimal revenues from product sales, and as of December 31, 2020, our accumulated deficit was $48.5 million. We expect that we will need to raise substantial additional funding in the future (see “Risk Factors – Risks Related to Our Financial Condition and Capital Requirements”).
In Europe, Singapore, India, Hong Kong, Russia, Thailand, Taiwan, South Africa, Mexico, Australia, Israel, Columbia and Costa Rica we have approvals for either our ProSense or our IceSense3 systems, or, in certain countries, both products. For instance, in China, we have approval for our IceSense3 (without the disposables) (see “Business – Our Products – Regulatory Approvals” below for additional information). In addition, in order to generate significant revenue, we are seeking to pursue additional regulatory approvals for our system for specific indications, in countries where we already have general regulatory approvals. In these countries, we are seeking regulatory approval for the treatment of specific tumors, including those found in breast, lungs, kidneys and bones. In addition, we are also seeking regulatory approvals for our system in other countries where we believe that there is significant potential for sales of our systems.
The procedure using our ProSense system begins with the introduction of our proprietary disposable probe into the tumor through a small pea-sized incision in the skin while the patient is under local anesthesia and/or sedation. The probe is guided by high-resolution ultrasound (for breast tumors) or computed tomography, or CT (for other indications). For some indications we use guiding needles (introducers) in order to guide the probe to the tumor. Once the probe is in place, LN2 is introduced into the probe in a closed-loop circuit, so that LN2 does not enter the body, and creates a freezing zone around the tip of the probe. During the freeze cycle, an ice ball forms in the freezing zone and encompasses the tumor, ablating the cancer or benign tumor, while the ice ball can be monitored by the physician using ultrasound or CT in order to avoid causing damage to the healthy tissue surrounding the tumor. Several minutes after the procedure is completed, the ice ball thaws and as a result thereof, there is no need for surgical removal of the dead tumor tissue, as the dead tissue is absorbed by the body in a natural process. The entire cryoablation procedure of freeze-thaw-freeze with our ProSense system generally takes between 15 to 40 minutes, depending on the size, type and location of the tumor. The same system configuration can be used and was designed to treat both malignant and non-malignant tumors. However, there is usually a different configuration for the probe handle between the systems used to treat breast tumors (with a primarily straight handle) and used to treat other indications (with a 90-degrees handle) due to the different imaging device that is used in each instance(see “Business – Our Products” for additional information). Pictured below is our system forming an ice ball (which in practice forms around the tissue within the body) in ultrasound gel.

61
As a minimally invasive alternative to surgery, cryoablation is much less traumatic than open surgery, and, based on current data, we believe this treatment is more affordable, entails less risk and generally results in fewer side effects and complications than open surgery. On the patient side, following procedures with our cryoablation technology, patients usually can resume normal activities within 24 hours after the procedure. In addition, in breast procedures with our technology, there is no need for a post-procedure reconstruction surgery. On the health provider side, procedures with our technology for breast tumors may be carried out in a clinic, while treatment by our ProSense system for other indications can generally be carried out as an outpatient procedure in a CT room. As a result, the potential profit margins for health care providers and payors are potentially greater than most of the current surgical procedures that are conducted in the operating room, which entail additional and expensive cost elements for the operating room and operating room staff. In addition, we believe that our LN2-based technology provides patients, medical service providers, physicians and insurers with advantages versus our competitors, especially those using heat to treat tumors, also known as thermal ablation. For example, the freezing effect on tissue from cryoablation produces less pain, and accordingly less anesthesia (which also reduces costs). A study published on April 8, 2021 by Elles M.F. van de Voort et al titled "Thermal Ablation as an Alternative for Surgical Resection of Small ( ≤2 cm) Breast Cancers: A Meta-Analysis" suggested that cryoablation has the lowest complication rate among breast cancer tumor procedures and the advantage of an analgesic effect. In addition, in comparison to the treatment of tumors with heat, when treating a tumor with cryoablation (unlike treatmesnt with heat technology), the freezing does not cause evaporation of the treated tissue and therefore provides the physician conducting the treatment with a clearer view of the tissue, which we believe enables the physician to carry out the procedure more accurately with a precise view of the tumor.
We believe that cryoablation has already started to be recognized for its true potential, and that it represents the future of treatment for certain benign and malignant tumors. Our ongoing business strategy, as further detailed below, is focused on helping us overcome certain factors that have limited our ability to generate significant revenues, including, but not limited to, obtaining support of key opinion leaders and leading medical associations for the use of cryoablation (and specifically, our systems) for the treatment of tumors and other indications. In recent years, and in part due to our efforts and accomplishments, cryoablation of malignant breast tumors has been recognized for its vast potential. For example, following preliminary results of our ICE3 trial, in October 2018, the ASBrS, which, among other things, sets guidelines for breast cancer treatment, updated its guidelines on performing cryoablation procedures on breast malignant tumors in their early stages. While not cited by the ASBrS, we believe that the results of our study were a factor in the ASBrS decision to update these guidelines. (see ”Business – Our Lead Indication and Market Opportunity – Primary Indication – Breast Tumors – Malignant Breast Tumors – Multi-Site Clinical Trial of the Cryoablation System ICE3 Study – United States” for additional information).
In addition to updating its guidelines, the ASBrS also recommend taking part in clinical trials and in registries for treating malignant breast tumors, each relating to cryoablation, to increase the knowledge and data of breast cancer cryoablation. Registries, by which uniform data is collected from patients through observational studies, represent an important stage in the commercialization of technologies and are part of the required procedure for receiving remuneration from health insurance companies.
Recent Developments
COVID-19 Pandemic
In December 2019, a novel strain of coronavirus, or COVID-19, was identified in Wuhan, China. The spread of COVID-19 from China to over 200 countries, including the United States and Israel, resulted in the World Health Organization declaring the outbreak of COVID-19 as a “pandemic,” or a worldwide spread of a new disease.
Due to the burden on health systems and the fear of infection, medical institutions in certain territories restricted elective procedures, including those related to our area of activity. As a result, certain of our plans for 2020 and our plans for 2021 were impacted, including, for example, our ability to hold face to face meetings, conventions and on-site trainings. In addition, although we were able to have certain face to face meetings and hold virtual meetings, our ability to reach new customers was impacted due to travel restrictions and social distancing requirements. We believe that due to the COVID-19 pandemic, investments in new technologies, including, for example, our ProSense system, decreased as customers sought to minimize their capital expenditures. Despite the resulting effects of the COVID-19 pandemic, we do not believe that we have sustained any material adverse effect on our financial condition and operations, and in spite of the COVID-19 pandemic, our revenues for our fiscal year ended December 31, 2020 increased by 138% in comparison to the year ended December 31, 2019 (see “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” for additional information). We continued to face challenges during the first quarter of 2021, and expect to continue to experience COVID-related challenges for the foreseeable future, mainly related to delays in shipments of materials by our suppliers, and disruption in access to new medical service providers and costumers due to the continued restrictions on international travel, direct access to medical service providers and public gatherings. Any disruption of suppliers or access to medical service provider would likely impact our progress, as well as our ability to access capital through the financial markets.
62
Although we were not able to increase sales to new customers as planned prior to the onset of the COVID-19 pandemic, we believe that certain competitive advantages that our ProSense system has versus surgical treatment helped us generate sales to our existing customers, and avert any material adverse effect on our financial condition or operations.
We continue to examine the consequences of the COVID-19 pandemic, perform risk assessments and implement operational solutions that we believe will help us deal with the COVID-19 pandemic, we are unable to accurately predict the impact that the COVID-19 pandemic will have on our operations going forward due to uncertainties that will be dictated by the length of time that the COVID-19 pandemic and related disruptions continue, the impact of governmental regulations that might be imposed in response to such pandemic and overall changes in the behavior of our customers.
Revenues and Growth Strategy
Our strategic objective is to be the leader in the field of cryoablation treatment for benign and malignant breast tumors and other interventional oncology indications. Using our innovative ProSense LN2 cryoablation system (and, in the future, potentially using additional systems) and propriety disposable probes and introducers, we intend to create a recurring revenues stream by selling single use probes to the users of our ProSense system. For tumors which are not in the breast, we also sell a disposable (single use) introducer which is used to assist with navigating the probe to the tumor.
Our revenues are based on a number of business models.
| ● | Sale of the system and disposables to distributors and/or to end users. |
| ● | Loan/Lease the system under an end user commitment to purchase a minimum number of disposables per month. |
In both models, although we may lease/sell a fixed number of systems over a period of time, the sales of the disposables are tied to the number of procedures conducted (similar to the razor/razor-blade model), and therefore, we expect to sell an increasing number of disposables as the number of procedures increases.
In order to generate significant revenues, we believe that we need to achieve each of the following milestones: (i) FDA approval for commercialization of our products for treatment of malignant tumors in breast, (ii) recommendations for the use of our products in guidelines of medical associations, such as the ASBrS, and (iii) the need to obtain category I CPT code and coverage for our products. We believe that reaching these milestones, while focusing on our business growth strategy, will help us achieve greater revenues. Our growth strategy includes the following actions.
| ● | Obtaining regulatory approval for our ProSense and other future systems in countries within which we do not currently have regulatory approval as well as obtaining regulatory approvals for additional indications in countries within which we already have certain approvals. Specifically, we intend to seek to obtain FDA approval to commercialize our products for the treatment of malignant breast tumors. |
| ● | Obtaining clinical data (by conducting both sponsored and independent clinical trials for our systems) and by gaining the support of these key opinion leaders. |
63
| ● | Expanding our distribution network for further commercialization, which may include distribution and/or license agreements or other forms of collaborative agreements. |
| ● | Obtaining the relevant approvals to allow for reimbursement to end-users of our systems. |
| ● | Having cryoablation treatment included in the recommendation guidelines as a valid treatment option of certain medical associations, such as the ASBrS. |
| ● | Continuing research and development efforts aimed at developing our next generation single Probe and MultiSense systems. We believe that completing development and obtaining regulatory approval to commercialize our next generation single Probe and MultiSense systems for a variety of indications, in the United States is necessary in order for us to grow our business. |
| ● | Expanding utilization and use of our products for breast cancer treatment by commercial sales and clinical studies around the world. |
Our Lead Indication and Market Opportunity
Our lead indication is breast tumors. There are generally two types of breast tumors: those that are non-cancerous, or benign, and those that are cancerous, or malignant.
Primary Indication
Breast Tumors
With regard to the costs involved in treating breast tumors (both for benign and malignant tumors), the National Cancer Institute estimates that national expenditure for breast cancer treatment increases each year. Thus, in 2010, the cost of treating breast cancer was approximately $16.5 billion in the United States – higher than any other type of cancer. This cost was expected to increase to $20.5 billion by 2020. Individual costs vary, depending on the stage of the malignancy and treatment options selected.
Benign Breast Tumors
The majority of breast tumors are benign (non-cancerous), and are generally not life-threatening compared to breast cancer. Fibroadenomas is a common type of benign breast tumors. They are solid benign (noncancerous) tumors common among women of various ages. Fibroadenoma tumors range in size and on average can be located anywhere in the breast, from the size of a marble to up to 2.5 centimeters in diameter, and tend to grow during pregnancy and breastfeeding. According to scientific publications from recent years, 10% of all women in the world suffer from fibroadenomas, and the phenomenon is particularly common among women under the age of 30 (80% of breast biopsies for women of these ages identify benign tumors). Many physicians and oncologists recommend removal of benign tumors for a variety of reasons, including: concerns that the tumor will increase in size, pain and due to concerns that the existence of a benign breast lump will make it difficult to manually discover breast cancer in the future. However, the vast majority of benign tumors are left untreated for reasons including cost and undesirable cosmetic outcomes, the latter of which, is generally not caused by treatment with our ProSense system.
Clinical Trial in Fibroadenoma (Benign Breast Tumors)
We sponsored and completed a prospective clinical trial in benign breast tumors in the Czech Republic, Israel and Germany. Between April 2009 and September 2012, data was collected from 60 procedures that were performed across four clinical sites located in Czech Republic, Israel and Germany (two sites). The IceSense3 was used to conduct the cryoablation procedures. The primary endpoint was to create an ice ball which would successfully engulf the entire tumor, as seen using ultrasound imaging. Our inclusion criteria were patients over 18 years old with core biopsy-proven breast fibroadenoma between 0.5 cm and 3.0 cm. The expected probability of success was 88%. At the one-year follow-up, in 93% of the cases, the fibroadenomas no longer existed. A June 2015 publication highlighting the data concluded that cryoablation of the fibroadenoma using a LN2 system demonstrated meaningful reduction in volume, palpability, pain and an improvement in cosmetic results from the procedure. No serious adverse events related to the IceSense3 system occurred during the clinical trial.
64
Malignant Breast Tumors
According to recent estimates from the American Breast Cancer Foundation’s publications, breast cancer is the most commonly diagnosed cancer among American women. Of the diagnosed breast cancers, it is estimated by the American Cancer Society that 73% of breast cancers are low-risk, while according to the American Society of Clinical Oncology, localized breast cancer accounts for 63% of total breast cancer incidence. In 2021, it is estimated that about 30% of newly diagnosed cancer in women will be breast cancer, resulting in approximately 281,550 new cases of invasive breast cancer in the United States. Furthermore, the American Breast Cancer Foundation estimates that the mortality rate in 2021 will be 43,600 women. It is further estimated that breast cancer is the second leading cause of cancer-related death in women (after lung cancer). Additionally, the American Cancer Society estimates that from 2008 to 2017 incidence rates have increased slightly by 0.5% per year. More recently, in February 2021, the American Breast Cancer Foundation reported that the average risk of a woman in the United States of developing breast cancer during her lifetime is approximately 13%, which means that there is a one (1) in eight (8) chance that a woman will develop breast cancer over the course of her life. A study conducted by the American Association for Cancer Research suggests that the number of breast cancer patients may increase by 50% by 2030, which shows a significant long-term market growth potential.
Traditional treatment options for malignant breast tumors include surgery, radiation, and chemotherapy. Concerning the surgical path, most breast cancer cases will require to have some type of surgery to remove the tumor. Surgical treatment often entails either breast-conserving surgery, or BCS, in which only the part of the breast containing the cancer is removed, or mastectomy, in which the entire breast is removed, including all of the breast tissue and sometimes also nearby tissue.
Clinical Trials in Cancerous (Malignant) Breast Tumors
We are currently sponsoring and/or participating in three clinical trials in malignant breast tumors spanning across the United States, Japan, Hong Kong and China.
Multi-Site Clinical Trial of the Cryoablation System ICE3 Study – United States
In May 2014, we initiated a multi-site clinical trial in the United States, which included participation of 19 sites in the ICE3 trial. The ICE3 trial was intended to expand the clinical base for using our cryoablation system for treating low-risk, small breast cancer tumors (up to 1.5 centimeters). The primary goal of ICE3 was to assess the effectiveness by the breast tumors local recurrence rates in patients who undergo cryoablation without excision for a follow-up period of five years. The inclusion criteria were patients over 65 years old with core biopsy-proven invasive ductal carcinoma with unifocal primary disease, tumor size less than 1.5 cm, Nottingham grade 1-2, estrogen and/or progesterone receptor positive and HER2 negative and breast size adequate for safe cryoablation.
In February 2019, the last patient was recruited. In total, 211 patients were recruited to the ICE3 trial, 206 of whom have enrolled, and 194 undergone Cryoablation according to the study protocol.
In April 2021, as part of the yearly conference of the ASBrS, the interim results of the ICE3 trial were presented. At the follow up period of 34.83 months following treatment with our cryoablation system, only 2.06% (four patients) experienced cancer recurrence. The 36-month local Failure Free Probability is 99.22%. The statistical analysis presented at the ASBrS conference by Dr. Richard Fine, an ICE3 investigator who serves as Program Director of the Breast Surgical Oncology Fellowship and as Director of Research and Education at the West Comprehensive Breast Center in Germantown, TN, indicates that among patients treated using our cryoablation system, the chance of non-recurrence in a population of patients with low-risk breast cancer, in early stages, and up to 1.5 cm tumor size, for a period of up to three years, is between 94.58%% and 99.89%%, with a statistical significance (confidence level) of 95%. Dr. Fine has also reported that freezing low risk breast tumors in the early stages delivers greater patient satisfaction at a lower cost than traditional interventions. No significant device-related adverse events were reported, and 95% percent of patients and 98% of treating physicians reported satisfaction with the cosmetic results.
We intend to continue monitoring patients for five years after undergoing our cryoablation treatment, and as such, the ICE3 trial is expected to be completed in 2024. In June 2021 we had 49 patients that have completed a five-year follow-up, 41 patients that have completed a four-year follow-up, 39 patients that have completed a three-year follow-up, and 51 patients that have completed a two-year follow-up. We believe that the interim results presented at ASBrS conference will assist us in the process of submitting an application for specific approval for breast cancer from the FDA.
65
Independent Clinical Trial of the Cryoablation System – Japan
Since May 2012, an independent clinical trial has been ongoing at the Kameda Medical Center in Kamogawa, Japan using our IceSense3 cryoablation system. So far, over 400 cryoablation procedures have been conducted using our cryoablation system (in addition to approximately 80 additional procedures with a different system). According to information reported on the International Cryosurgery Society Convention, which was held in September 2019, in 304 of the 400 patients who were treated with cryoablation between 2006 and 2019, the recurrence rate of breast cancer was lower than 1%. The inclusion criteria were patients with histologically-proven breast cancer at stage 0 (TisN0M0) or stage 1 (T1N0M0) with tumor mass and lesion (including the progress within intraductal component) less than 1.0 cm. The primary endpoints were comparing the tumor necrosis rate, cosmetic results of the procedure, clarity of imaging, safety and therapeutic effects of IceSense3 versus a competing cryoablation device.
In addition, since May 2018, an independent investigator initiated clinical trial in Japan at the St. Marianna University School of Medicine Department of Mammary Gland and Endocrine Surgery using our ProSense system. The trial’s purpose was conducted in breast cancer tumors of up to 1.5 cm (in their early stages). The inclusion criteria were patients with histologically-proven breast cancer at stage 0 (TisN0M0) or stage 1 (T1N0M0), between the age of 20 and 85 years, HER2 protein expression-negative status, Ki-67 positivity of ≤ 20% and lesion spread of 1.5 cm or less. The trial was completed after the enrollment of seven patients, in which pathology was confirmed using a vacuum-assisted biopsy. In all seven patients, there was no evidence of malignant cells, and following a monitoring period of two years, all seven patients remain disease free with no clinical and imaging evidence. The primary endpoint of this clinical trial was to expand the clinical knowledge of cryoablation of cancerous breast tumors and enable to implement this technology at the St. Marianna University Hospital as a routine procedure. Based on these results, the trial received approval from the hospital to offer cryoablation for breast cancer in a commercial setting.
Independent Clinical Trial of the Cryoablation System –Hong Kong and China
Since November 2018, an independent clinical trial has been ongoing at the Queen Mary Medical Center in Hong Kong and at Hong Kong-Shenzhen University Hospital in China. According to information disclosed by the primary investigator, treatments using our ProSense cryoablation system has been performed to date in 20 patients, out of an expected total patient pool of 150, in which each patient underwent an excision after the cryoablation to examine the ablated area by pathology. Following the first phase, the remaining patients are expected to be monitored according to the standard of care without excision. The purpose of this clinical trial is to expand the clinical knowledge of cryoablation of cancerous breast tumors. The primary endpoints were to demonstrate cryosurgery’s efficacy in ablating small breast cancers, safety with low morbidity and the use of PET/MRI as an effective imaging modality to assess post-treatment responses. The inclusion criteria were patients between 18 and 70 years old with core biopsy-proven T0/T1a and b breast cancer or ductal carcinoma in situ.
Other Clinical Indications
We are targeting other tissue tumor ablation for our ProSense system, in organs such as: lungs, kidneys, bones and other organs. Our approach to these indications is to collaborate with hospitals and doctors to conduct clinical trials in order to gain additional information regarding the potential of our products to treat certain diseases. While our cryoablation products have been approved by various regulatory agencies for variety of oncology and surgical uses, we will need to validate our products in specific indications, and in certain situations in order to obtain specific regulatory approvals, and/or to collect medical data, which will be required in order to successfully market our products for these indications.
Lung Cancer
The American Cancer Society has estimated that in 2021, approximately 235,760 new cases of lung and bronchus cancer will be diagnosed in the United States while an estimated of 131,880 patients will die of such cancers in the United States during 2021.
Lung Cancer Clinical Trial
Since November 2013, an independent clinical trial in cryoablation of lung tumors in non-small cell carcinoma or metastatic lesions has been ongoing at the Kameda Medical Center in Kamogawa, Japan using our IceSense3 system. Based on data provided to us, this trial is an ongoing trial, and to date, more than 300 procedures have been performed using our cryoablation system.
In November 2020, the lead investigator for the trial published the results in a peer reviewed article in the European Journal of Radiology. The results in the published article covered the cryoablation treatments in 101 patients during the years 2013 through 2019. The patients in this review were divided into four categories based on the size of maximum tumor diameter, as follows: (1) group 1: tumor size up to 0.9 cm; (2) group 2: tumor size between 1 and 1.2 cm; (3) group 3: tumor size between 1.3 and 1.7 cm; and (4) group 4: tumor size larger than 1.8 cm. Ten patients experienced local recurrences. There were no recurrences in groups 1 or 2 (0%). There was one recurrence in group 3 (4%) and nine in group 4 (33%), indicating local control to be better in smaller tumors (p<0.001). The 3-year recurrence-free survival rates were: 86% in group 1; 97% in group 2; 93% in group 3; and 53% in group 4, indicating survival to be better in smaller tumors (p<0.001). There were no serious adverse events reported.
66
The results of the trial, as disclosed in the published article, were that the treatment of tumors in groups 1 and 2 through cryoablation and LN2and local control of the tumor and the lack recurrence of the cancer was more effective than in groups 3 and 4. As part of the trial, tumors treated in groups 1 and 2 did not have local recurrence, while in groups 3 and 4, 4% and 33%, respectively, of the tumors had local recurrence. The 3-year recurrence-free survival rates were: 86% in group 1; 97% in group 2; 92% in group 3; and 53% group 4, indicating the survival to be better in smaller tumors (p < 0.001). No patient had treatment-related mortality. The most frequent complication was pneumothorax, with a rate of 24%, while the decrease of pulmonary function was just 3%.
The peer reviewed publication also highlighted that the use of cryoablation treatment with only one needle for the majority of the patients in the trial represented an advantage in comparison to systems that use argon gas, which usually requires the use of 2-3 needles for a procedure on the same tumors size. We believe that the publication of the results of the trial in a peer reviewed publication raises the validity of using our products for the treatment of lung tumors.
Kidney Tumors
The American Cancer Society has estimated that in 2021, approximately 76,080 new cases of kidney and renal pelvis cancer will be diagnosed in the United States, while an estimated of 13,780 patients will die of such cancers in the United States during 2021.
According to an article published in the Journal of Endourology in 2016, the cost of cryoablation of a kidney is about 53% of the cost of an open kidney surgery, also known as an open partial nephrectomy, and 51% of a robot-assisted partial nephrectomy. Despite percutaneous cryoablation being performed in older patients with higher comorbidity indices, percutaneous cryoablation had lower hospitalization times and eliminated the cost of many hospital-related expenses such as room and board.
Kidney Tumors Clinical Trials
In 2012, a clinical trial was initiated at Bnei Zion Medical Center in Haifa, Israel in collaboration with the Shamir/Assaf Harofeh Medical Center in Be’er Ya’akov, Israel. Procedures were performed using our ProSense system for freezing and ablating kidney tumors. The recruitment for this trial is completed.
As of January 2021, our IceSense3 and ProSense systems have been used to freeze and ablate kidney tumors in 120 patients as planned in the protocol. Initial results of the clinical trial were also presented in March 2019, as part of the annual conference of the European Association of Urology, held in Barcelona, Spain, which demonstrated treatment of an aggregate of 45 tumors in sizes of up to 4 cm in 42 patients, which had undergone a follow-up of at least 12 months (the average follow-up period was 18.2 months). According to the initial results, the recurrence rate is 7%. In addition, the average cryoablation time was 22 minutes and the average length of the entire treatment was 50.5 minutes per patient. One serious adverse event was reported in this trial.
Our Products
Existing Products
Our ProSense system is our second generation cryoablation system comprised of two main parts, the main cryoablation console system, our unique disposable probe, and the associated disposable introducer (guiding needle), which is used for procedures which are not in the breast. Our first product, the IceSense3 was initially designed for cryoablation of breast tumors. After identifying a number of additional possible applications for the use of our technology, such as treating lungs, kidneys and bones, and other possible applications, in 2016 we launched our ProSense system as well as associated disposables (which, as show below, are inserted into the body to conduct the freezing process).
67
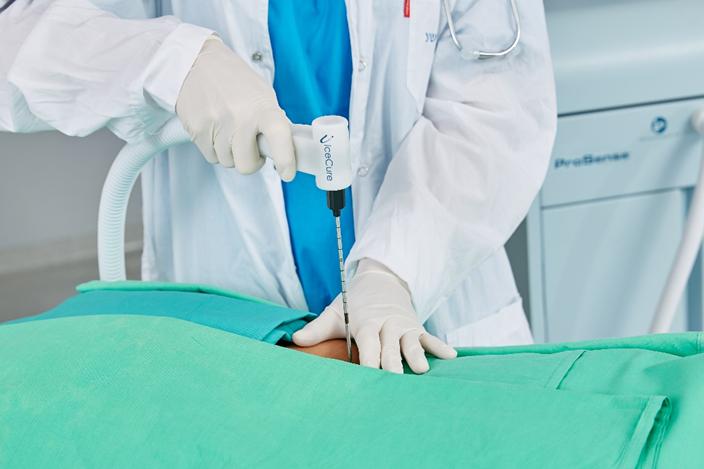
The following illustration demonstrates the course of the cryoablation procedure with our ProSense system in an ultrasound of the breast:
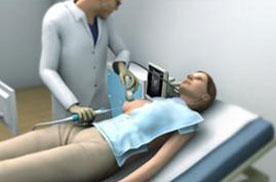 |
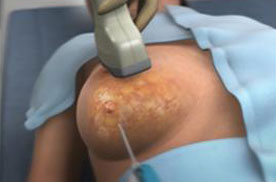 |
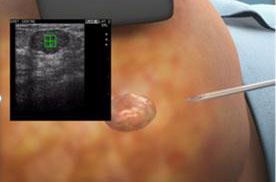 |
Locating the procedure in ultrasound imaging and planning the probe insertion path. |
Inserting the probe to the tumor through the selected path, using real-time ultrasound imaging. | |
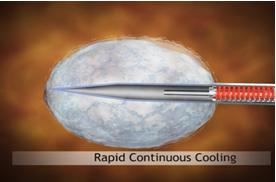 |
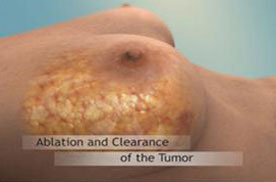 |
 |
| Freezing the tumor while creating an ice ball, and then heating the needle for easy removal. | The freezing immediately destroys the tumor tissue. | After the treatment, patients are left without scars and the dead tissues are absorbed into the body. |
68
Research and Development
While we are currently commercializing our single probe ProSense system and its disposables, we are also in the process of developing our next generation single probe system. In addition, we are also focusing on developing our next generation MultiSense system. We started development of our first-generation system for technical proof concept process and evaluation of clinical application in 2006. In the beginning of 2009, we started to develop our IceSense3 system, which was later improved and replaced by the ProSense. Both the IceSense3 and ProSense are commercially available systems which include technical modifications to our first generation system in order to perform clinical procedures. We started developing our MultiSense in 2016 which we did not commercialize.
We intend to commercialize our next generation systems, which will be our third-generation systems, subject to regulatory approvals. Our strategy is to make our next generation systems more efficient and user-friendly in several aspects. We believe that the next generation single probe system will have better performance which will allow it to create a bigger ice ball. The physical size of the system will have a smaller footprint, which is an important factor in CT rooms and clinics. Since our next generation MultiSense system will have more than one probe, it will have freezing capabilities that both our current and next generation systems do not have, such as the ability to treat tumors in more than one location simultaneously. We believe that our next generation MultiSense system will help us to increase our ability to penetrate the non-breast tumors market more easily and allow us to offer a more innovative product.
We are continuing our research and development efforts to complete development of both the next generation single probe system and the MultiSense system and intend to commence commercialization, subject to applicable regulatory approval, in the future. We currently expect to complete development of the next generation single probe system for limited release by the second quarter of 2022 and our MultiSense system during the first quarter of 2024. Even if we complete development as planned, we do not yet know if and when we will begin to commercialize these systems or whether commercialization of such systems will lead to us generating increased revenue.
Regulatory Approvals
We have received a broad range of regulatory approvals for our products in the United States, Europe, China (IceSense3 system only), Singapore, Hong Kong, Mexico, Australia, Israel, Columbia, Costa Rica, India (only for disposables (see “– India” below for additional information), Thailand, Russia, South Africa and Taiwan. Moreover, we are pursuing additional regulatory approvals in other indications in existing countries and in other countries where we believe that there is significant potential for sales. For example, Japan (for specific approval in certain indications), China (for disposables and for our ProSense system, which is an upgrade to the already approved IceSense3) and the United States (for specific approval for breast cancer).
We market our ProSense for a specific indication per the rules and medical device classification in the specific territory.
United States
In the United States, we received from the U.S. Food and Drug Administration, or FDA, 510(k) approval for our IceSense3, ProSense and MultiSense and the related disposables. On December 10, 2007, we received initial 510(k) clearance for our IceSense3 system for ablation indications specific to urology, oncology, dermatology, gynecology, general surgery, thoracic surgery and proctology. On November 29, 2010, we received 510(k) clearance for our IceSense3 system for the ablation of breast fibroadenomas under general surgery. On December 20, 2019, we received 510(k) approval to allow us to market and sell our IceCure family (which includes Icesense3, ProSense and MultiSense) systems for the treatment of breast fibroadenomas, prostate and kidney tissue, liver metastases, tumors, skin lesions, and other indications, and treat our products as “one family of products,” which means that any additional approval given by the FDA in relation to the family of products will apply automatically to the “family” as one product, without the need for FDA approval for each separate system; provided, however, that we expect to require individual approvals for our products from the FDA if we seek marketing approval for a new specific indication, as is the case for the use of these products for breast cancer.
On December 31, 2020, we submitted a pre-submission package to the FDA for approval of breast cancer indication, based on our ICE3 trial interim results as published on for our ProSense and MultiSense systems. As part of the pre-submission package, we requested that we receive approval for this indication through the 510(k) submission pathway or De Novo classification. There can be no guarantee that the FDA approves the use of any of our products for the treatment of this indication, or, even if approval is given to market our products, there can be no guarantee that the approval is given via the 510(k) pathway or De Novo classification, which could result in additional costs and a longer timeline until we receive any such approval. If we do not receive approval via the 510(k) pathway or De Novo classification, we may seek to receive a PMA. See “Government Regulation – PMA Pathway” for additional information. We intend to continue to work closely with the FDA in hopes of receiving specific approval for breast cancer cryoablation, which we believe will help us grow our commercialization efforts in the United States.
On March 31, 2021, we were granted Breakthrough Device Designation, or BDD, from the FDA for our 510(k) approved ProSense, and proposed indication for use, including for use in the treatment of patients with T1 invasive breast cancer and/or patients not suitable for surgical alternatives for the treatment of breast cancer.
Europe – CE Mark
In Europe, we received Conformitè Europëenne, or CE, mark approval for our ProSense cryoablation system and its disposables with indications for use as a cryosurgical tool in the fields of general surgery, dermatology, thoracic surgery, gynecology, oncology, proctology and urology, which enable us to sell our ProSense in order to perform procedures in the indications we aim to such as breast cancer, Kidney, lung, bone and other indications.
69
Europe, South Africa and Asia
In Europe, Singapore, Hong Kong and South Africa, our ProSense system has specific approval for cryoablation of benign and malignant tumors in the breast, lung, bone and liver. In China, only our IceSense3 is approved for use, without probes or other disposables.
In China, the IceSense3 console has National Medical Products Administration, or NMPA (formerly the China Food and Drug Administration, or CFDA) approval. We have submitted for an additional five-year renewal. We are also working on the IceSense3 application to add disposables. We, also working on a new application for our ProSense and disposables. Until we receive a NMPA approval for our disposables, we will only be able to sell the IceSese3 system and provide, free of charge, the disposables solely for non-commercial purposes, such as research and training for medical teams.
In Japan, our ProSense and disposables are not yet approved by the Japanese regulatory authority, the Pharmaceuticals and Medical Devices Agency, or PMDA. We started to sell our products in Japan as “experimental products” under “private doctor importation” licenses in low quantities. Currently, without approval from the PMDA, we are only able to sell a limited number of ProSense systems and disposables under “private doctor importation” licenses. Following our distribution agreement with Terumo, Terumo will be responsible, and bare all costs of obtaining regulatory approval for breast cancer from the PMDA to sell our products in Japan.
In Thailand, our products have the Ministry of Public Health approval for our ProSense console and associated disposables to treat malignant breast tumors and other intended uses.
Israel
In Israel, we have received the Medical Devices and Disposables, or AMAR, authorization for use of our freezing technology for freezing of benign and malignant tumors, including and among others, breast, lungs, bones, kidney and other indications which enables us to market our products and will allow doctors in Israel to use our product for those indications listed above.
Russia and Taiwan
In 2020, we received regulatory approvals to market and distribute ProSense and disposables in Russia and Taiwan for use of our products in the treatment of benign and cancerous tumor cells through freezing in a number of organs, such as kidneys, lungs, liver and bones.
India
In 2020, we received regulatory approval to commercialize our disposables in India, where our systems themselves do not require regulatory approval before commercialization.
Commercialization
We began selling our IceSense3 and disposable probes in small quantities, for cryoablation of fibroadenoma in the United States in 2011. As of 2012, we began selling all of our products (IceSense3, ProSense and disposables) in the United States and other countries, with sales generated primarily by our sponsored ICE3 trial and independent clinical trials not sponsored by us. Following limited commercialization efforts that took place during our research and development phase, in 2018 and 2019, we started to increase our commercial efforts to sell our products to distributors and end users in the United States, Europe and Asia. During the year ended December 31, 2020, we continued our commercialization efforts, although they were impacted by COVID-19. Although we expect the ongoing COVID-19 pandemic to continue to impact our customers’ inclination to increase capital expenditures, we intend to continue to commercialize our products during 2021 and beyond.
As part of our strategy of raising awareness for our products and proprietary technologies within the medical community, although no formal agreement exists, in light of the ASBrS’ importance in our industry, we are operating in the United States with them as the leading organization that sets guidelines for breast cancer treatment. We are also continually seeking to collaborate with key opinion leaders in additional territories such as Italy, France, Hong Kong, Japan and Germany in order to create awareness and collect clinical evidence for our technology in such territories.
In addition, in the United States, we are working with leading breast surgeons and breast interventional radiologists in order to initiate collaborations in the field of freezing malignant breast tumors. At the Radiological Society of North America conference held in 2018, our freezing technology was declared one of 15 groundbreaking solutions in this field. In the United States, unlike in other states, as described below, we are entering a consolidated market and, in addition to our existing growth strategy, we will need to refine our marketing approach in order to generate significant revenue.
We currently have distribution agreements in Japan and Singapore, Thailand, Germany, Italy, France, Spain, Hungary, South Africa, Australia, Hong Kong, Taiwan, India, UAE and other countries. We believe that the following are our material distribution agreements.
70
In August 2019, we entered into an exclusive distribution agreement, or the Terumo Japan Agreement, for licensing, registration, import, marketing, sale, promotion and distribution of our ProSense system and its disposable products with breast tumors, with a leading global medical company, Terumo Corporation, or Terumo, in Japan and Singapore; provided, however, that with respect to the exclusivity clause, (i) we shall continue to have the ability to sell our system and disposables in Japan until Terumo obtains regulatory permit for marketing and distribution of our ProSense system and (ii) notwithstanding the foregoing, the exclusivity condition shall continue in force only for so long as Terumo purchases a minimum agreed upon number of products during the term of the Terumo Japan Agreement. We believe that the Terumo Japan Agreement will accelerate the commercialization of our ProSense system and associated disposables to treat malignant breast tumors in Japan and Singapore. The Terumo Japan Agreement requires Terumo to obtain necessary regulatory permits for marketing and distribution in Japan and obtaining reimbursement approvals. In Singapore, we have received the applicable regulatory approvals for our products.
The Terumo Japan Agreement is for an initial term of five years from the date of receipt of regulatory approvals for the sale of the Company’s products in Japan and is automatically extended for additional terms of five years each, unless a party notifies the other party of its intention to terminate the Terumo Japan Agreement at least one year prior to the end of the term, or at any time upon the mutual agreement of the parties in writing. A party shall have the right to terminate the Terumo Japan Agreement upon a breach of a material provision of the Terumo Japan Agreement by the other party or upon the insolvency of such other party, subject to certain conditions. In addition, the Terumo Japan Agreement may be terminated by either party under certain terms, including the option of revocation by the Company if Terumo does not purchase at least 60% of the minimum quantities defined in the Terumo Japan Agreement for the purchase of products and if Terumo fails to obtain the regulatory approvals on the dates stipulated in the Terumo Japan Agreement. In some cases, upon revocation or termination of the agreement, Terumo will assign to the Company the regulatory filings and regulatory approvals for the marketing and distribution of the ProSense system in Japan.
The minimal aggregate consideration that Terumo owes us under the Terumo Japan Agreement is approximately $13.2 million, of which, we have received $4 million as proceeds for distribution rights, sharing clinical data, the first purchase order, and another $341 thousand for sale of products. Our revenues pursuant to the Terumo Japan Agreement amounted to $348,000 in 2019 and $1,721,000 in 2020.
In December 2020, we entered into an exclusive distribution agreement with Terumo Thailand (of Terumo), or the Terumo Thailand Agreement, for licensing, registration, import, marketing, sale, promotion and distribution of ProSense system and its disposables, in Thailand. The exclusivity condition shall continue in force only for so long as Terumo Thailand purchases a minimum agreed upon number of products during the term of the agreement. The distribution agreement is intended to accelerate the commercialization of our ProSense system and associated disposables to treat malignant and benign tumors in the breast, kidney, lung and other applications in Thailand. The agreement requires Terumo Thailand to obtain necessary regulatory permit for marketing and distribution in Thailand.
The Terumo Thailand Agreement is for an initial term of six years and is automatically extended for additional terms of six years each, unless a party notifies the other party of its intention to terminate the Terumo Thailand Agreement at least one year prior to the end of the term, or at any time upon the mutual agreement of the parties in writing. A party shall have the right to terminate the Terumo Thailand Agreement upon a breach of a material provision of the Terumo Thailand Agreement by the other party or upon the insolvency of such other party, subject to certain conditions. In addition, the Terumo Thailand Agreement may be terminated by either party under certain terms, including the option of revocation by the Company if Terumo does not purchase at least 60% of the minimum quantities defined in the Terumo Thailand Agreement for the purchase of products, and the option of revocation by Terumo Thailand if the Company discontinues its business relating to the ProSense system and its disposables or does not bring action with respect to an infringement of the exclusive distribution right within a reasonable time frame.
To date, we have received up-front payments in a total aggregate amount of $300 thousand under the Terumo Thailand Agreement. The minimal aggregate consideration that Terumo Thailand owes us under the agreement is approximately $7.2 million, of which $450,000 is to be paid in three equal instalments of $150,000 for the sole distribution rights and knowledge sharing and $329,000 for the first purchase order. As of July 6, 2021, we have received the $300,000 instalment for the sole distribution rights and $107,000 as part of the first purchase order.
Our primary customers are hospitals, interventional radiological centers, ambulatory centers and private clinics that purchase, lease or loan the ProSense system and buy the disposables directly from us, as well as distributors who sell medical products and procedures in our field of activity. The primary users of our products are breast surgeons and interventional radiologists. In some instances, we also place our ProSense in hospitals and clinics in return for a commitment by the hospital or clinic to purchase a minimum number of probes per month for an extended period. As we sell more cryoablation systems, we anticipate the volume of sales of our probes will materially increase as we engage in a razor/razor blades sales model (see “Business – Revenues and Growth Strategy” for additional information).
In addition to our distribution agreements and other aspects of our product revenues and growth strategies, as described below, commercialization of our products is also highly dependent on the receipt of Current Procedural Terminology, or CPT, characterization. On July 2, 2019, our application for a CPT category III codes (0581T) describing the use of cryoablation for treating cancerous breast tumors was approved by the American Medical Association. We intend to pursue additional CPT category codes for breast cancer cryoablation, which is required in order to make procedures with our products eligible for reimbursement from insurance companies in the United States. Even if we are successful in obtaining approval for our products for entry into additional CPT category codes, these changes generally take over 12 months to go into effect, usually at the start of a new calendar year.
In order to cause our products to receive entry into additional CPT categories, we intend to work with the ASBrS and conduct registry trials to collect additional data that we believe will support the use of our system and technology as a viable treatment option for breast cancer. We believe that by conducting such trials and collecting such data, which will result in increased use of our products, and potentially additional publications regarding their use, the ASBrS may amend its guidelines and to enable our treatment system to receive CPT I approval, which could enable medical providers to receive appropriate reimbursement for treatment through our systems. At this time, it is unlikely that our ProSense (or any future system) will be eligible for rebates from insurance companies and other third-party payors without specific regulatory approvals for specific indications. Specific indications such as kidney cancer, liver cancer, bone tumors (under cryoanalgesia) have CPT category I codes and reimbursement in a level of US$ 5,000-$9,000 per case. At this time, we have yet to initiate any marketing efforts for these indications.
71
Intellectual Property
Our intellectual property portfolio consists of 28 issued patents (17 in the United States, 5 in Europe, 5 in China and 1 in Hong Kong), as further detailed in the table below. In addition to our issued patents, we also have one patent application (application No. 16/785,686), which we filed with the U.S. PTO in February 2020 for a cryogenic pump, a utility patent, which has an expected expiration date of February 10, 2040.
| Patent No. | Application No. | Title | Type of Patent Protection | Expiration Date | Country | PCT No. | ||||||||
| 7967815 | 12/731,219 | Cryosurgical Instrument with Enhanced Heat Transfer | Utility patent | 03/25/2030 | US | PCT/US2011/025663 | ||||||||
| 102378600 | 201180000141.8 | Utility patent | 02/22/2031 | China | ||||||||||
| EP2549941 | 2549941 | Utility patent | 02/22/2031 | Great Britain | ||||||||||
| 2549941 | Utility patent | 02/22/2031 | France | |||||||||||
| 602011054052.1 | Utility patent | 02/22/2031 | Germany | |||||||||||
| 502019000004651 | Utility patent | 02/22/2031 | Italy | |||||||||||
| 7967814 | 12/700,761 | Cryoprobe with Vibrating Mechanism | Utility patent | 02/05/2029 | US | N/A | ||||||||
| 8162812 | 12/722,845 | Combined Cryotherapy and Brachytherapy Device and Method | Utility patent | 03/12/2029 | US | N/A | ||||||||
| 7938822 | 12/778,172 | Heating and Cooling of Cryosurgical Instrument Using a Single Cryogen | 05/12/2030 | US | PCT/US2011/031722 | |||||||||
| 103079487 | 201180022782.3 | Utility patent | 04/08/2031 | China | ||||||||||
| EP2533716 | 2533716 | Utility patent | 04/08/2031 | Great Britain | ||||||||||
| 2533716 | Utility patent | 04/08/2031 | France | |||||||||||
| 602011018919.0 | Utility patent | 04/08/2031 | Germany | |||||||||||
| 502015000073978 | Utility patent | 04/08/2031 | Italy | |||||||||||
| 8080005 | 12/846,047 | Closed Loop Cryosurgical Pressure and Flow Regulated System | Utility patent | 06/10/2030 | US | N/A | ||||||||
| 103096824 | 201180043677.8 | Cryosurgical Instrument for Treating Large Volume of Tissue | Utility patent | 09/01/2031 | China | PCT/US2011/050214 | ||||||||
| EP2593028 | 2593028 | Utility patent | 09/01/2031 | Great Britain | ||||||||||
| 2593028 | Utility patent | 09/01/2031 | France | |||||||||||
| 602011040633.7 | Utility patent | 09/01/2031 | Germany | |||||||||||
| 502017000124372 | Utility patent | 09/01/2031 | Italy | |||||||||||
| 8591505 | 13/339,506 | Cryosurgical Instrument with Redirected Flow | Utility patent | 05/19/2031 | US | PCT/US2011/067858 | ||||||||
| 103402449 | 201180068737.1 | Utility patent | 12/29/2031 | China | ||||||||||
| EP2683315 | 2683315 | Utility patent | 12/29/2031 | Great Britain | ||||||||||
| 2683315 | Utility patent | 12/29/2031 | France | |||||||||||
| 602011031296.0 | Utility patent | 12/29/2031 | Germany | |||||||||||
| 502016000113273 | Utility patent | 12/29/2031 | Italy | |||||||||||
72
| 8083733 | 12/988,233 | Cryosurgical Instrument with Enhanced Heat Exchange | Utility patent | 12/27/2019 | US | PCT/IB2009/051532 | |||||||
| 7137978 | 10/637,904 | Cryosurgical Instrument and its Accessory System | Utility patent | 12/02/2023 | US | N/A | |||||||
| 7481806 | 11/531,058 | Cryosurgical Instrument and its Accessory System | Utility patent | 08/11/2023 | US | N/A | |||||||
| 7731711 | 12/336,866 | Cryosurgical Instrument and its Accessory System | Utility patent | 08/11/2023 | US | N/A | |||||||
| 7425211 | 11/462,244 | Cryogenic Probe for Treating Large Volume of Tissue | Utility patent | 11/24/2026 | US | N/A | |||||||
| 7803154 | 11/832,778 | Cryogenic Probe for Treating Large Volume of Tissue | Utility patent | 11/24/2026 | US | N/A | |||||||
| 8709005 | 13/232,203 | Coiled Heat Exchanger for Cryosurgical Instrument | Utility patent | 12/10/2031 | US | PCT/US2011/051529 | |||||||
| 103442657 | 201180069176.7 | Utility patent | 09/14/2031 | China | |||||||||
| 1190057 | 14103188.0 | Utility patent | 09/14/2031 | Hong Kong | |||||||||
| EP2696785 | 2696785 | Utility patent | 09/14/2031 | Great Britain | |||||||||
| 2696785 | Utility patent | 09/14/2031 | France | ||||||||||
| 602011031962.0 | Utility patent | 09/14/2031 | Germany | ||||||||||
| 502016000122670 | Utility patent | 09/14/2031 | Italy | ||||||||||
| 9050075 | 14/133980 | Utility patent | 05/11/2031 | US | |||||||||
| 8906004 | 14/204,175 | Coiled Heat Exchanger for Cryosurgical Instrument | Utility patent | 05/11/2031 | US | N/A | |||||||
| 9808302 | 15/125,258 | Phase Separation of Cryogen in Cryosurgical Instrument | Utility patent | 05/11/2031 | US | PCT/US2014/064292 | |||||||
| 9039689 | 14/547,483 | Phase Separation of Cryogen in Cryosurgical Instrument | Utility patent | 01/28/2026 | US | N/A |
Our intellectual property covers our technological platform, as well as innovative developments that will be used in our future products. Our patent number 8083733 relating to cryosurgical instrument with enhanced heat exchange expired in 2019 and our patents number 7137978, 7481806 and 7731711, relating to Cryosurgical instrument and its accessory system are scheduled to expire in 2023. These patents are not currently used, and are not expected to be used, by the Company for the development of our current and future technology and products and we do not expect the expiry and pending expiry to influence our business.
73
In addition, to our patent portfolio, we have the following issued trademarks. In addition, we have a number of other trademarks in the United Kingdom that we intend on abandoning.
| Registration No. | Application No. | Expiration Date | Country | Mark | Renewal Due Date | |||||
| 4063706 | 77/615,741 | N/A | US | ICECURE® | 11/29/2021 (10 year) | |||||
| 4146269 | 85/430,438 | N/A | US | ICECURE LOGO | 05/22/2022 (10 year) | |||||
| 017884253 | 04/04/2018 | N/A | Europe | ICECURE LOGO | 04/04/2027 (10 year) | |||||
| 5251758 | 86/790,477 | N/A | US | PROSENSE® | 07/25/2023 (5 year) | |||||
| 017884265 | 17884265 | N/A | Europe | PROSENSE® | 04/04/2028 (10 year) | |||||
| 4029030 | 77/215,497 | N/A | US | ICESENSE® | 09/20/2021 (10 year) | |||||
| 27566828 | 27566828 | N/A | China | PROSENSE | 02/06/2029 (10 year) | |||||
| 27566823 | 27566823 | N/A | China | PROSENSE (CHINESE) | 11/13/2027 (10 year) | |||||
| 010241305 | 010241305 | N/A | Europe | ICECURE | 09/05/2021 (10 year) | |||||
| 010241297 | 010241297 | N/A | Europe | ICESENSE3 | 09/05/2021 (10 year) | |||||
| 910241263 | 010241263 | N/A | Europe | ICESENSE | 09/05/2021 (10 year) | |||||
| 018042365 | 018042365 | N/A | Europe | ICECURE (NEW LOGO) | 03/28/2029 (10 year) | |||||
| 6301784 | 2019-125291 | N/A | Japan | PROSENSE | 10/08/2030 (10 year) | |||||
| 6301783 | 2019-125290 | N/A | Japan | ICECURE (ENGLISH) | 10/08/2030 (10 year) | |||||
| 6301782 | 2019-125289 | N/A | Japan | ICECURE (Logo) | 10/08/2030 (10 year) |
Production and Manufacturing
The majority of the manufacturing of the ProSense console’s components is outsourced, and we complete the final assembly in our facility in Israel. The majority of the manufacturing of our disposables, including sterilization and packing is outsourced. We conduct the final inspections at our facility.
The various components of our ProSense and probes are purchased and manufactured by several vendors and subcontractors. We are engaged with approximately 90 suppliers of components for our ProSense and MultiSense systems and its disposables. The primary suppliers for our ProSense and MultiSense systems and disposables are Sinai Technologies Metal Manufacturing LTD, J.H. Avidan LTD and Concept Group LLC.
We consistently monitor our inventory levels, manufacturing and distribution capabilities, and maintain recovery plans to address potential disruptions that we may encounter. In the future, as we scale up our sales and production further, we may implement a turnkey operation with select manufacturers for our probes.
We enter into agreements with our vendors and subcontractors. Pursuant to such agreements, the Company will provide the parts and raw materials and the subcontractor will provide the components and/or perform the assembly of such components and/or service in accordance with specific terms of the mutually agreed work instructions and purchase orders. The agreements define the responsibilities of each party and the regulatory and compliance requirements that apply and contain industry-standard terms and guidelines.
Competition
The medical device and tumor treatment industry is characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Cryoablation is an alternative approach to surgically removing tumors and/or to heat ablation of tumors, such as radiofrequency ablation, microwave and high intensity focused ultrasound. We encounter significant competition across our product lines in each market in which we sell our products from various companies, some of which may have greater financial and marketing resources than we do. We also face competition from non-medical device companies, as pharmaceutical companies, which may offer alternative therapies and treatments. We believe that the ability of our products and services to deliver rapid minimally-invasive treatments in-office or ambulatory hospital settings serves as a key competitive advantage versus surgical and other tumor treatment solutions. In the current environment of managed care, with economically-motivated buyers, consolidation among healthcare providers, increased competition and declining reimbursement rates, we have been increasingly required to compete on the basis of price, value, reliability and efficiency, which we believe we have been able to do and hope to continue to do.
74
We believe that our cryoablation technology, and especially our LN2-based technology, has advantages over heat ablation technologies, which include, but are not limited to the below competitive advantages relative to heat ablation technologies.
| ● | Pain: Because of the freezing effect on tissue, our procedure is less painful then heat ablation. |
| ● | Anesthesia: Due to the lower amount of pain that is generally caused by procedures from cryoablation, patients generally require less anesthesia. |
| ● | Accuracy: Image guided visualization of the cryoablation ice ball is clearer than heat ablation as the freezing does not cause evaporation, thereby allowing the cryoablation to be more accurate. |
We believe that our direct competitors for cryoablation of malignant breast tumors are Sanarus Medical, Inc., part of Hologic Inc. and Galil Medical Ltd., part of Boston Scientific Corporation. Sanarus Medical, Inc. also uses LN2-based technology.
For tumors in other organs, other alternatives to surgical removal or cryoablation are available, such as thermal ablation, (including, radio frequency ablation, microwave ablation and high intensity focused ultrasound). Our primary direct competitors also include other cryoablation companies, such as EndoCare, Inc., part of Siemens Healthineers AG and Hygea Medical Technology Co. Ltd. for interventional radiology. Hygea Medical Technology Co. Ltd. is a company that also uses LN2-based technology. Unlike these competitors who have a multi probe system in the market, we are still developing our MultiSense system. Despite this, we believe that our LN2-based cryoablation technology is superior over our competitors, including Galil Medical and EndoCare, for a variety of reasons, including the following:
| ● | our cryoablation LN2 technology allows deeper freezing temperature, up to -160° Celsius, which results in a faster and more efficient destruction of the tumor cells; |
| ● | our LN2 technology allow us to achieve lower temperatures faster, thereby resulting in a shorter procedure; |
| ● | our effective ablating zone for one probe is greater than that of technologies utilizing argon-based technology probes (we are able to use one probe for a three-centimeter tumor while argon-based technologies need more than one probe to create the same killing zone; |
| ● | Using one probe in our technology is less complicated and easier to most physicians; |
| ● | the price of argon gas is significantly higher and less available in some territories than LN2 which makes our procedures more cost effective; and |
| ● | argon gas is stored in a 4,800 PSI gas balloons, which potentially creates a risk of explosion whereas our LN2 is stored in low pressure containers which presents less risk and allows for office setting procedures. |
We believe that these technological advantages will enable us to compete effectively with our competitors. In addition, we believe that by completing the development, and initiating commercialization of our next generation MultiSense system will enable us to compete even more effectively with our competitors.
Government Regulation
Our products and business are subject to extensive federal, state, local and foreign laws and regulations, including those relating to the protection of the environment, health and safety and our failure to comply with applicable requirements could harm our business. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change, or new laws may be enacted.
Both federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other things, the billing practices of healthcare providers and the marketing of healthcare products.
75
We believe that we have structured our business operations and relationships with our distributors and customers to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise. In addition, because there is a risk that our products are used off label, we believe we are subject to increased risk of prosecution under these laws and by these entities even if we believe we are acting appropriately. We discuss below the statutes and regulations that are most relevant to our business and most frequently cited in enforcement actions.
FDA Regulation of Medical Devices
The Federal Food, Drug and Cosmetic Act, or FDCA, and FDA regulations establish a comprehensive system for the regulation of medical devices intended for human use. Our products include medical devices that are subject to these regulations, as well as other federal, state, local and foreign, laws and regulations. The FDA is responsible for enforcing the laws and regulations governing medical devices in the United States.
The FDA classifies medical devices into one of three classes (Class I, Class II, or Class III) depending on their level of risk and the types of controls that are necessary to ensure device safety and effectiveness. The class assignment is a factor in determining the type of premarketing submission or application, if any, that will be required before marketing in the United States.
| ● | Class I devices present a low risk and are not life-sustaining or life-supporting. The majority of Class I devices are subject only to “general controls” (e.g., prohibition against adulteration and misbranding, registration and listing, good manufacturing practices, labeling, and adverse event reporting. General controls are baseline requirements that apply to all classes of medical devices.) | |
| ● | Class II devices present a moderate risk and are devices for which general controls alone are not sufficient to provide a reasonable assurance of safety and effectiveness. Devices in Class II are subject to both general controls and “special controls” (e.g., special labeling, compliance with performance standards, and post market surveillance. Unless exempted, Class II devices typically require FDA clearance before marketing, through the premarket notification (510(k) process.) | |
| ● | Class III devices present the highest risk. These devices generally are life-sustaining, life-supporting, or for a use that is of substantial importance in preventing impairment of human health, or present a potential unreasonable risk of illness or injury. Class III devices are devices for which general controls, by themselves, are insufficient and for which there is insufficient information to determine that application of special controls would provide a reasonable assurance of safety and effectiveness. Class III devices are subject to general controls and typically require FDA approval of a premarket approval, or PMA, application before marketing. |
Unless it is exempt from premarket review requirements, a medical device must receive marketing authorization from the FDA prior to being commercially marketed, distributed or sold in the United States. The most common pathways for obtaining marketing authorization are 510(k) clearance and PMA.
All of our medical device products sold in the United States are subject to regulation as medical devices under the FDCA, as implemented and enforced by the FDA. The FDA governs the following activities that we perform or that are performed on our behalf, to ensure that medical products we manufacture, promote and distribute domestically or export internationally are safe and effective for their intended uses:
| ● | product design, preclinical and clinical development and manufacture; |
| ● | product premarket clearance and approval; |
| ● | product safety, testing, labeling and storage; |
| ● | record keeping procedures; |
| ● | product marketing, sales and distribution; and |
| ● | post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions and repair or recall of products. |
76
510(k) Pathway
The 510(k) review process compares a new device to a legally marketed device. Through the 510(k) process, the FDA determines whether a new medical device is “substantially equivalent” to a legally marketed device (i.e., predicate device) that is not subject to PMA requirements. “Substantial equivalence” means that the proposed device has the same intended use as the predicate device, and the same or similar technological characteristics, or if there are differences in technological characteristics, the differences do not raise different questions of safety and effectiveness as compared to the predicate, and the information submitted in the 510(k) application demonstrates that the proposed device is as safe and effective as the predicate device.
To obtain 510(k) clearance, a company must submit a 510(k) application, containing sufficient information and data to demonstrate that its proposed device is substantially equivalent to a legally marketed predicate device. These data generally include non-clinical performance testing (e.g., software validation, animal testing electrical safety testing), but may also include clinical data. Typically, it takes three to twelve months for the FDA to complete its review of a 510(k) submission; however, it can take significantly longer and clearance is never assured. During its review of a 510(k) application, the FDA may request additional information, including clinical data, which may significantly prolong the review process. After completing its review of a 510(k) application, the FDA may issue an order, in the form of a letter, that finds the device to be either (i) substantially equivalent and states that the device can be marketed in the United States, or (ii) not substantially equivalent and states that device cannot be marketed in the United States. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device.
After a device receives 510(k) clearance or de novo classification, any modification that could significantly affect the safety or effectiveness of the device, or that would constitute a major change in its intended use, including significant modifications to any of our products or procedures, requires submission and clearance of a new 510(k) application or de novo classification or approval of a PMA. The FDA relies on each manufacturer to make and document this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. Modifications meeting certain conditions may be candidates for a streamlined FDA review known as Special 510(k) review, which the FDA intends to process within 30 days of receipt. If a device modification requires the submission of a 510(k) application, but the modification does not affect the intended use of the device or alter the fundamental technology of the device, then summary information that results from the design control process associated with the cleared device can serve as the basis for clearing the application. A Special 510(k) allows a manufacturer to declare conformity to design controls without providing new data. When a modification involves a change in material, the nature of the “new” material will determine whether a traditional or Special 510(k) is necessary. An Abbreviated 510(k) is another type of 510(k) process that is intended to streamline the review of data through the reliance on one or more FDA-recognized consensus standards, special controls established by regulation, or FDA guidance documents. In most cases, an Abbreviated 510(k) includes one or more declarations of conformity to an FDA-recognized consensus standard. We may also make minor product enhancements that we believe do not require new 510(k) clearances. If the FDA disagrees with our determination regarding whether a new 510(k) clearance was required for these modifications, we may need to cease marketing and/or recall the modified device. The FDA may also subject us to other enforcement actions, including, but not limited to, issuing a warning letter or untitled letter to us, seizing our products, imposing civil penalties, or initiating criminal prosecution.
De Novo Pathway
Medical device types that the FDA has not previously classified as Class I, II, or III are automatically classified as Class III regardless of the level of risk they pose. The Food and Drug Administration Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the de novo classification procedure. This procedure allows a manufacturer whose novel device is automatically classified as Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. Prior to the enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, in July 2012, a medical device could only be eligible for de novo classification if the manufacturer first submitted a 510(k) premarket notification and received a determination from the FDA that the device was not substantially equivalent. FDASIA streamlined the de novo classification pathway by permitting manufacturers to request de novo classification directly without first submitting a 510(k) premarket notification to the FDA and receiving a not substantially equivalent determination. We originally obtained marketing authorization for our system using the de novo classification process after receiving a not substantially equivalent determination following the submission of a 510(k) premarket notification. We have subsequently used the 510(k) clearance process to obtain authorization from the FDA for changes to our marketed system.
77
PMA Pathway
Unlike the comparative standard of the 510(k) pathway and the De Novo Pathway, the PMA approval process requires an independent demonstration of the safety and effectiveness of a device. PMA is the most stringent type of device marketing application required by the FDA. PMA approval is based on a determination by the FDA that the PMA contains sufficient valid scientific evidence to ensure that the device is safe and effective for its intended use(s). A PMA application generally includes extensive information about the device including the results of clinical testing conducted on the device and a detailed description of the manufacturing process.
After a PMA application is accepted for review, the FDA begins an in-depth review of the submitted information. FDA regulations provide 180 days to review the PMA and make a determination; however, in reality, the review time is normally longer (e.g., one to three years). During this review period, the FDA may request additional information or clarification of information already provided. In addition, during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the data supporting the application and provide recommendations to the FDA as to whether the data provide a reasonable assurance that the device is safe and effective for its intended use. The FDA generally will conduct a preapproval inspection of the manufacturing facility to ensure compliance with Quality System Regulation, or QSR, which imposes comprehensive development, testing, control, documentation and other quality assurance requirements for the design and manufacturing of a medical device.
Based on its review, the FDA may (i) issue an order approving the PMA, (ii) issue a letter stating the PMA is “approvable” (e.g., minor additional information is needed), (iii) issue a letter stating the PMA is “not approvable,” or (iv) issue an order denying PMA. A company may not market a device subject to PMA review until the FDA issues an order approving the PMA. As part of a PMA approval, the FDA may impose post-approval conditions intended to ensure the continued safety and effectiveness of the device including, among other things, restrictions on labeling, promotion, sale and distribution, and requiring the collection of additional clinical data. Failure to comply with the conditions of approval can result in materially adverse enforcement action, including withdrawal of the approval.
Most modifications to a PMA approved device, including changes to the design, labeling, or manufacturing process, require prior approval before being implemented. Prior approval is obtained through submission of a PMA supplement. The type of information required to support a PMA supplement and the FDA’s time for review of a PMA supplement vary depending on the nature of the modification.
Breakthrough Devices Program
The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the FDA’s mission to protect and promote public health.
The Breakthrough Devices Program offers manufacturers an opportunity to interact with the FDA’s experts through several different program options to efficiently address topics as they arise during the premarket review phase, which can help manufacturers receive feedback from the FDA and identify areas of agreement in a timely way. Manufacturers can also expect prioritized review of their submission.
In addition, the United States’ Centers for Medicare and Medicaid Services, or CMS, finalized a new Medicare coverage pathway in efforts to bring new and innovative technologies to beneficiaries sooner. If a product is granted BDD, the company can approach the MCIT, and start the process of accelerating the coverage of new, innovative breakthrough devices to Medicare beneficiaries. If successful, National Medicare coverage under the MCIT pathway could begin immediately upon the date of FDA market authorization (that is, the date the medical device receives PMA; 510(k) clearance; or the granting of a De Novo classification request) for the Breakthrough Device or on the date designated by the manufacturer within any point during the four-year eligibility period for coverage under MCIT. The Biden administration put a hold on all MCIT related rules that hadn’t gone into effect yet, and as a result thereof, until there is a decision on this matter, we are not able to make a request for coverage under any MCIT pathway.
As part of the Breakthrough Device Program, the MCIT, which is pending approval by the Biden Administration, could provide national Medicare coverage for our ProSense, for a four-year duration starting on the day of the FDA marketing authorization. The Company will still be required to apply for CPT1 codes under regular approval procedures in order to receive reimbursement after the four-year provisional coverage period.
78
Clinical Trials
A clinical trial is typically required to support a PMA application or de novo classification, and is sometimes required for a 510(k) pre-market notification. Clinical trials of medical devices in the United States are governed by the FDA’s Investigational Device Exemption, or IDE, regulation. This regulation places significant responsibility on the sponsor of the clinical study including, but not limited to, choosing qualified investigators, monitoring the trial, submitting required reports, maintaining required records, and assuring investigators obtain informed consent, comply with the study protocol, control the disposition of the investigational device, submit required reports, etc.
Clinical trials of significant risk devices (e.g., implants, devices used in supporting or sustaining human life, devices of substantial importance in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health) require FDA and Institutional Review Board, or IRB, approvals prior to starting the trial. FDA approval is obtained through submission of an Investigational Device Exemption, or IDE, application. Clinical trials of non-significant risk, or NSR, devices, (i.e., devices that do not meet the regulatory definition of a significant risk device) only require IRB approval before starting. The clinical trial sponsor is responsible for making the initial determination of whether a clinical study is significant risk or NSR; however, IRB and/or FDA reviewer may review this decision and disagree with the determination.
An IDE application must be supported by appropriate data, such as performance data, animal and laboratory testing results, showing that it is safe to evaluate the device in humans and that the clinical study protocol is scientifically sound. There is no assurance that submission of an IDE will result in the ability to commence clinical trials. Additionally, after a trial begins, the FDA may place it on hold or terminate it if, among other reasons, it concludes that the clinical subjects are exposed to an unacceptable health risk.
As noted above, the FDA may require a company to collect clinical data on a device in the post-market setting.
The collection of such data may be required as a condition of PMA approval. The FDA also has the authority to order, via a letter, a post-market surveillance study for certain devices at any time after they have been cleared or approved.
Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the United States. Similarly, in Europe the clinical study must be approved by a local ethics committee and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, regardless of its classification or premarket pathway, numerous additional FDA requirements generally apply. These include, but are not limited to:
| ● | Establishment registration and device listing requirements; | |
| ● | QSR, which governs the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of finished devices; | |
| ● | Labeling requirements, which mandate the inclusion of certain content in device labels and labeling, and generally require the label and package of medical devices to include a unique device identifier, and which also prohibit the promotion of products for uncleared or unapproved, i.e., “off-label,” uses; | |
| ● | Medical Device Reporting, or MDR, regulation, which requires that manufacturers and importers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and | |
| ● | Reports of Corrections and Removals regulation, which requires that manufacturers and importers report to the FDA recalls (i.e., corrections or removals) if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; manufacturers and importers must keep records of recalls that they determine to be not reportable. |
79
The FDA enforces these requirements by inspection and market surveillance. Failure to comply with applicable regulatory requirements can result in an enforcement action by the FDA, which may include, but is not limited to, the following sanctions:
| ● | untitled letters or warning letters; | |
| ● | fines, injunctions and civil penalties; | |
| ● | recall or seizure of our products; | |
| ● | operating restrictions, partial suspension or total shutdown of production; | |
| ● | refusing our request for 510(k) clearance or premarket approval of new products; | |
| ● | withdrawing 510(k) clearance or premarket approvals that are already granted; and | |
| ● | criminal prosecution. |
We are subject to unannounced device inspections by the FDA, as well as other regulatory agencies overseeing the implementation of and compliance with applicable state public health regulations. These inspections may include our suppliers’ facilities. We cannot assure that we have adequately complied with all regulatory requirements or that one or more of the referenced sanctions will not be applied to us as a result of a failure to comply.
International Regulation
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. In order to market our products in other countries, we must obtain regulatory approvals and comply with extensive safety and quality regulations in other countries. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ. The European Union/European Economic Area (the “EU/EEA”), requires a CE conformity mark in order to market medical devices. Many other countries, such as Australia, India, New Zealand, Pakistan and Sri Lanka, accept CE or FDA clearance or approval, although others, such as Brazil, Canada, China and Japan require separate regulatory filings.
In the EU and the EEA, devices are required to comply with the essential requirements of the EU Medical Devices Directive being replaced by Medical Device Regulation (MDR 2017/745) which will allow marketing medical devices, in EU, under MDD until 26 May 2024. Compliance with these requirements would entitle us to affix the CE conformity mark to our medical devices, without which they cannot be commercialized in the EU and EEA. To demonstrate compliance with the essential requirements and obtain the right to affix the CE conformity mark we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I), where the manufacturer can issue a CE Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the Medical Devices Directive, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization accredited at the European Commission to conduct conformity assessments. The Notified Body would typically audit and examine the quality system for the manufacture, design and final inspection of our devices before issuing a certification demonstrating compliance with the essential requirements. Based on this certification we can draw up a CE Declaration of Conformity which allows us to affix the CE Mark to our products.
On April 5, 2017, the European Parliament passed the Medical Devices Regulation, which repeals and replaces the EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations would be directly applicable (i.e., without the need for adoption of EEA member State laws implementing them) in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and in vitro diagnostic devices and ensure a high level of safety and health while supporting innovation.
80
The Medical Device Regulation was meant to become applicable three years after publication (in May 2020). However, on April 23, 2020, to allow EEA national authorities, notified bodies, manufacturers and other actors to focus fully on urgent priorities related to the COVID-19 pandemic, the European Council and Parliament adopted Regulation 2020/561, postponing the date of application of the Medical Device Regulation by one year (to May 2021). Once applicable, the new regulations will among other things:
| ● | strengthen the rules on placing devices on the market and reinforce surveillance once they are available; | |
| ● | establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; | |
| ● | improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; | |
| ● | set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and | |
| ● | strengthen rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
These modifications may have an impact on the way we design and manufacture products and the way we conduct our business in the EEA. We are progressing in our plans to meet the new requirements.
Further, the advertising and promotion of our products in the EU and the EEA is subject to the laws of individual EU and EEA member states implementing the EU Medical Devices Directive, Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other EU and EEA member state laws governing the advertising and promotion of medical devices. These laws may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals.
We are in the process of implementing the new MDR requirements to conform with the European requirements.
We have received AMAR approval in Israel. In addition, we received approval from the MedCert Zertifizierungs und Prufungsgsesellschaft fur die Medizin GmbH of Germany, and are entitled to print the CE Mark on our products, after having examined the EU Technical File for each new product.
Other Regulatory Matters
Manufacturing, sales, promotion and other activities following product approval are also subject to regulation by numerous regulatory authorities in addition to the FDA, including, CMS, other divisions of the Department of Health and Human Services, the Department of Justice, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety and Health Administration, the Environmental Protection Agency, and state and local governments. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Manufacturing, sales, promotion and other activities are also potentially subject to federal and state consumer protection and unfair completion laws.
The distribution of medical device products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of medical device products.
81
Third-Party Payors Coverage and Reimbursement
Procedures using our products to treat breast cancer are not reimbursed currently by private or governmental third-party payors in the United States. We intend to seek reimbursement through private and governmental third-party payors in the future, although significant uncertainty exists as to whether coverage and reimbursement of such procedures will be approved. In both the United States and foreign markets, our ability to expand utilization of the system and to attract commercialization partners depends, in part, on the availability of adequate coverage and reimbursement from third-party payors, including, in the United States, governmental payors such as the Medicare and Medicaid programs, and private health insurers. Medicare is a federally funded program managed by the CMS, through local contractors that administer coverage and reimbursement for certain healthcare items and services furnished to the elderly and disabled. Medicaid is an insurance program for certain categories of patients whose income and assets fall below state defined levels and who are otherwise uninsured, that is both federally and state funded and managed by each state. The federal government sets general guidelines for Medicaid and each state creates specific regulations or other guidelines that govern its individual program. Each payor, whether governmental or private, has its own process and standards for determining whether it will cover and reimburse a procedure or a particular product. Since private payors often rely on the lead of the governmental payors in rendering coverage and reimbursement determinations, achieving favorable CMS coverage and reimbursement is often a significant gating issue for successful introduction of a new product. The competitive position of our systems will depend, in part, upon the extent of coverage and adequate reimbursement for the procedures in which such products are used.
Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage policies and reimbursement rates are attained for procedures using our products, less favorable coverage policies and reimbursement rates may be implemented in the future.
State and federal healthcare reform measures may be adopted in the future, any of which may result in additional reductions in Medicare and other healthcare funding, and otherwise affect the prices we may obtain for any of our products for which we may obtain regulatory approval, or the frequency with which any such products is prescribed or used.
In addition, in some foreign countries, the proposed pricing for a medical device must be approved before it may be lawfully marketed. The requirements governing medical device pricing vary widely from country to country. In some countries, we may be required to conduct a clinical study or other studies that compare the cost-effectiveness of any of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Historically, products launched in the EU do not follow price structures of the United States and generally tend to be significantly lower. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If pricing is set at unsatisfactory levels or if reimbursement of our products is unavailable or limited in scope or amount, our revenues from sales by us or our distributors and the potential profitability of any of our product candidates in those countries could be negatively affected.
Other Healthcare Laws and Compliance Requirements
Healthcare providers, physicians, and third-party payors will affect the utilization of any products for which we obtain marketing approval. Our future arrangements with healthcare providers and physicians may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any device for which we obtain marketing approval. In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the CMS, other divisions of the United States Department of Health and Human Services (e.g., the Office of Inspector General), the United States Department of Justice and individual United States Attorney offices within the Department of Justice, and state and local governments. The applicable laws and regulations include the federal Anti-Kickback Statute, the False Claims Act, and the Health Insurance Portability and Accountability Act of 1996, or HIPAA.
| ● | The Anti-Kickback Statute makes it illegal for any person, including a device manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration, directly or indirectly, in cash or in kind, that is intended to induce or reward referrals, including the purchase, recommendation, or order of a particular device, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by up to five years in prison, criminal fines, administrative civil money penalties and exclusion from participation in federal healthcare programs. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it. |
82
| ● | The Federal False Claims Act imposes civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities (including manufacturers) for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or avoiding, decreasing or concealing an obligation to pay money to the federal government. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties of between $10,957 and $21,916 for each separate false claim and the potential for exclusion from participation in federal healthcare programs. Conduct that violates the False Claims Act also may implicate various federal criminal statutes. The government may deem manufacturers to have “caused” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Claims which include items or services resulting from a violation of the federal Anti-Kickback Statute also are deemed false or fraudulent claims for purposes of the False Claims Act. Our future marketing and activities relating to the reporting of wholesaler or estimated retail prices for our products and other information affecting federal, state and third-party reimbursement for our products, and the sale and marketing of our product and any future product candidates, are subject to scrutiny under this law. | |
| ● | HIPAA which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, also imposes certain obligations, including contractual terms and technical safeguards, with respect to maintaining the privacy, security and transmission of individually identifiable health information. | |
| ● | HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. | |
| ● | The Federal Physician Payments Sunshine Act within the Affordable Care Act, and its implementing regulations, which requires that certain manufacturers of devices and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members; and | |
| ● | Analogous state and foreign fraud and abuse laws and regulations, such as anti-kickback and false claims laws, which may apply to sales and marketing arrangements and claims involving healthcare items or services reimbursed by any third party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts. Such laws are generally broad and are enforced by various state agencies and private actions. |
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information, which are applicable to “business associates”—independent contractors or agents of HIPAA covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity.
Current and future legislation
In the United States and foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or any collaborators, may receive for any approved products.
83
U.S. Regulation
The Affordable Care Act has had, and is expected to continue to have, a significant impact on the healthcare industry. The Affordable Care Act was designed to expand coverage for the uninsured while at the same time containing overall healthcare costs. With regard to pharmaceutical products, among other things, the Affordable Care Act expanded and increased industry rebates for drugs covered under Medicaid programs and made changes to the coverage requirements under the Medicare prescription drug benefit. There remain judicial, Congressional and executive branch challenges to certain aspects of the Affordable Care Act, and we expect there will be additional challenges and amendments to the Affordable Care Act in the future. While Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the Affordable Care Act such as removing or delaying penalties, starting January 1, 2019, for not complying with the Affordable Care Act’s individual mandate to carry health insurance, delaying the implementation of certain Affordable Care Act-mandated fees, and increasing the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D. Additionally, on December 15, 2018, a Texas U.S. District Court Judge ruled that the Affordable Care Act is unconstitutional in its entirety because the individual mandate was repealed by Congress. Further, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. On March 2, 2020, the United States Supreme Court granted the petitions for writs of certiorari and held oral arguments on November 10, 2020. Accordingly, we continue to evaluate the effect that the Affordable Care Act has on our business. Other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. For example, through the process created by the Budget Control Act of 2011, there are automatic reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments, will remain in effect through 2030 unless additional Congressional action is taken. However, the Coronavirus Aid, Relief and Economic Security Act, or CARES Act, which was signed into law in March 2020 and is designed to provide financial support and resources to individuals and businesses affected by the COVID-19 pandemic, suspended the 2% Medicare sequester from May 1, 2020 through December 31, 2020, and extended the sequester by one year, through 2030. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers. In addition, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which have resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump administration’s budget proposals for fiscal year 2021 includes a $135 billion allowance to support legislative proposals seeking to reduce drug prices, increase competition, lower out-of-pocket drug costs for patients, and increase patient access to lower-cost generic and biosimilar drugs. In addition, the Trump administration previously released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out-of-pocket costs of drug products paid by consumers. The Department of Health and Human Services, or HHS, has solicited feedback on some of these measures and implemented others under its existing authority. On July 24, 2020 and September 13, 2020, President Trump announced several executive orders related to prescription drug pricing that seek to implement several of the administration’s proposals. The FDA also released a final rule on September 24, 2020 providing guidance for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. The likelihood of implementation of any of the other Trump administration reform initiatives is uncertain, particularly in light of the recent change in U.S. administration. In the coming years, additional legislative and regulatory changes could be made to governmental health programs that could significantly impact pharmaceutical companies and the success of our product candidates. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. The Affordable Care Act, as well as other federal, state and foreign healthcare reform measures that have been and may be adopted in the future, could harm our future revenues. Further, it is also possible that additional governmental action is taken in response to the COVID-19 pandemic.
84
Additional laws and regulations governing international operations
Since we further expand our operations outside of the United States, we must dedicate additional resources to comply with numerous laws and regulations in each jurisdiction in which we plan to operate. The Foreign Corrupt Practices Act, or FCPA, prohibits any United States individual or business from paying, offering, authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the United States, or the sharing with certain non-United States nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. Our presence outside of the United States, requires dedicating additional resources to comply with these laws, and these laws may preclude us from developing, manufacturing, or selling certain products and product candidates outside of the United States, which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial civil and criminal penalties and suspension or debarment from government contracting. The Securities and Exchange Commission, or the SEC, also may suspend or bar issuers from trading securities on the United States exchanges for violations of the FCPA’s accounting provisions.
Post-Marketing Regulations
Following clearance or approval of a new product, a company and the product are subject to continuing regulation by the FDA and other federal and state regulatory authorities, including, among other things, monitoring and recordkeeping activities, reporting to applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting for uses or in patient populations not described in the product’s approved labeling (known as “off-label use”), limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available products for off-label uses, manufacturers may not market or promote such off-label uses. Modifications or enhancements to the products or labeling or changes of site of manufacture are often subject to the approval of the FDA and other regulators, which may or may not be received or may result in a lengthy review process.
Government Grants
Our research and development efforts were financed in part through royalty-bearing grants from the Israel Innovation Authority, or the IIA. As of December 31, 2020, we have received the aggregate amount of approximately $2.46 million (including accumulated interest) from the IIA for the development of our products. With respect to such grants, we are committed to pay certain royalties up to the total grant amount, including accumulated interest. As of 31 December 2020, we paid approximately $269 thousand. Regardless of any royalty payment, we are further required to comply with the requirements of the Research Law, with respect to those past grants. When a company develops know-how, technology or products using IIA grants, the terms of these grants and the Research Law restrict the transfer of such know-how, and the transfer of manufacturing or manufacturing rights of such products, technologies or know-how outside of Israel, without the prior approval of the IIA. This may restrict our ability to move the production of our products outside of Israel, or to sell intellectual property and other know-how.
85
The royalty rate we have undertaken to pay the IIA is 3.5%, and in any event up to the level of the grant, including accumulated interest, being linked to the exchange rate of the U.S. dollar and bearing Libor interest. Starting from the second half of 2017, new directives were introduced, according to which small companies (up to an annual turnover of $70 million) are to pay royalties of 3%.
The total sum of royalties, including accumulated interest, we are required to repay the IIA, as of December 31, 2020 is approximately $2.2 million, net, after deducting the sums we paid as royalties to the IIA.
Organizational Structure
We have three wholly-owned subsidiaries: IceCure Medical Inc., IceCure Medical HK Limited and IceCure (Shanghai) MedTech Co., Ltd., a Chinese company fully owned by the subsidiary in Hong Kong.
IceCure Medical Inc. is our wholly-owned subsidiary incorporated in the State of Delaware. IceCure Medical Inc. is engaged in business development, marketing, managing clinical trial and selling our products in the United States.
IceCure Medical HK Limited is our wholly-owned subsidiary incorporated in Hong Kong. IceCure Medical HK Limited serves as a holding company for Icecure (Shanghai) MedTech Co., Ltd. Currently, there is no other activity in IceCure Medical HK.
IceCure (Shanghai) MedTech Co., Ltd., a subsidiary fully owned by IceCure Medical HK Limited., started its operations in 2021, and is expected to be engaged in obtaining regulatory approvals, business development, marketing, and selling our products in China.
Properties and Facilities
Our headquarters are located at 7 Ha’Eshel St., Caesarea, 3079504, Israel, where we currently occupy approximately 581 square meters (approximately 6,254 square feet). We lease our facilities and our lease ends on July 2022, extendable to July 2025.
Employees
As of the date of this prospectus, we have five senior management positions, all of whom are engaged on a full-time basis, and all of whom are engaged as employees. In addition to our senior management, we have 42 full-time employees and 11 part-time employees. The majority of our employees are located in Israel.
Our employees are not represented by labor unions or covered by collective bargaining agreements. We believe that we maintain good relations with our employees. However, in Israel, we are subject to certain Israeli labor laws, regulations and national labor court precedent rulings, as well as certain provisions of collective bargaining agreements applicable to us by virtue of extension orders issued in accordance with relevant labor laws by the Israeli Ministry of Economy and which apply such agreement provisions to our employees even though they are not part of a union that has signed a collective bargaining agreement.
All of our employment and consulting agreements include employees’ and consultants’ undertakings with respect to non-competition and assignment to us of intellectual property rights developed in the course of employment and confidentiality. With respect to our employees in Israel, the enforceability of such provisions is limited by Israeli law.
Legal Proceedings
On July 5, 2021, we were informed that a motion to certify a claim as a class action, or the Motion, was filed in Israel with the Tel Aviv District Court by Amir Yosef Brot, who claims to be a shareholder of the Company, or the Plaintiff. The Plaintiff’s claim is against the Company, the members of the board of directors, the controlling shareholder and the investors who took part in the private placement that was approved by our shareholders on March 7, 2021. In the Motion, the Plaintiff claims, inter alia, that we conducted a private placement of securities to the controlling shareholder and the investors at a significant discount to the Company’s share price at the time, that the share price did not reflect material information that was allegedly in the Company’s possession and which was also brought to the attention of the investors, and that there were alleged defects in the manner of approving the private placement by our shareholders. After a preliminary review of the Motion(without its annexes, since the motion has not yet been served on the Company), we believe that the Motion is without merit and that the factual description and the data underlying the Motion are incorrect and/or imprecise and we intend to defend ourselves vigorously.
In October 2018, the Israel Securities Authorities, or the ISA, commenced administrative enforcement proceedings against Wize Pharma Ltd., or Wize, Ron Mayron (who, at the relevant times, served as Chairman of Wize’s board of directors), and Wize’s Chief Financial Officer. In August 11, 2019, the ISA’s Administrative Enforcement Committee approved a settlement agreement pursuant to which Mr. Mayron undertook to pay a civil penalty of NIS 150,000 (equivalent to approximately $45,000) to the ISA. It should be clarified that Mr. Mayron resigned from his position as Chairman of Wize on November 7, 2018 and that Mr. Mayron was not barred from continuing to serve as an officer for public companies, including serving as the Company’s Chairman. As of today, the fine has been paid in full and Mr. Mayron is not subject to any sanctions or legal proceedings.
86
Directors and Senior Management
The following table sets forth information regarding our executive officers, key employees and directors as of July 6, 2021:
| Name | Age | Position | ||
| Ron Mayron | 58 | Chairman of the Board of Directors | ||
| Eyal Shamir | 60 | Chief Executive Officer, Director | ||
| Ronen Tsimerman | 51 | Chief Financial Officer, Chief Operation Officer | ||
| Shay Levav | 44 | Vice President, Regulatory and Quality Assurance and Clinical Applications | ||
| Tlalit Bussi Tel-Tzure | 49 | Vice President, Business Development and Global Marketing | ||
| Naum Muchnik | 44 | Vice President, Research, Development and Engineering | ||
| Doron Birger (1)(2)(4)(5) | 70 | Director | ||
| Yang Huang | 42 | Director | ||
| Sharon Levita (1)(2)(3)(4)(5) | 53 | Director | ||
| Oded Tamir (1)(2)(3)(4)(5) | 65 | Director |
| (1) | Member of the Compensation Committee |
| (2) | Member of the Audit Committee and Financial Statement Examination Committee |
| (3) | External Director (as defined under Israeli law) |
| (4) | Independent Director (as defined under Israeli law) |
| (5) | Independent Director (as defined under Nasdaq Stock Market rules) |
Ron Mayron, Chairman of the Board of Directors
Mr. Ron Mayron has served as Chairman of our board of directors since December 2017. Mr. Mayron has served as chairman of the board of directors of Resymmetry Ltd. since July 2016, InnoCan Pharma Corporation (CSE: INNO, FWB: IP4, OTC: INNPF) since November 2017 and Virility Medical LTD since October 2019, and as a member of the board of directors of BioLight Life Sciences Investments Ltd. (TASE: BOLT) since August 2015, G-Med Ltd. since September 2015, Kaizen Bio-Tec Ltd. since May 2017, Simplivia Ltd. since May 2019 and Kadimastem LTD (TASE: KDST) since December 2020. Mr. Mayron has also served as the founder and chief executive officer of RonMed Ltd. Prior to that, Mr. Mayron has served as chairman of the board of directors of Wize Pharma Inc. (OTC: WIZP) from April 2015 to October 2018 and Ocon Medical Ltd from January 2015 to November 2016, and as a member of the board of directors of EclipeIR (USA) Inc from June 2016 to September 2019. Mr. Mayron has also served in various positions at Teva Pharmaceutical Industries Ltd. (NYSE: TEVA, TASE: TEVA) from 1993 to 2014, including as vice president –Israel and Africa and chief executive officer of Teva Israel from 2009 to 2013. Mr. Mayron received his B.Sc. in industrial and management engineering from Ben-Gurion University of the Negev, Israel and MBA from Tel-Aviv University, Israel. Mr. Mayron also completed a special senior management and global leadership programs at the Massachusetts Institute of Technology (M.I.T), Boston and managerial skills for international business and executive international marketing programs at Insead University, France.
87
Eyal Shamir, Chief Executive Officer and Director
Mr. Eyal Shamir has served as our Chief Executive Officer since September 2016 and on our board of directors since December 2017. Mr. Shamir has over 15 years of experience as chief executive officer of medical device companies. He has served as chief executive officer of Erika Carmel Ltd. from May 2013 to August 2016, Tadbik Pack Ltd. from January 2011 to December 2012 and Hanita Lenses Ltd. from 2006 to 2010. Mr. Shamir received his B.A. in economics and business management from the Hebrew University, Israel and his MBA from the College of Management Academic Studies, Israel.
Ronen Tsimerman, Chief Financial Officer and Chief Operation Officer
Mr. Ronen Tsimerman has served as our Chief Financial Officer since May 2017 and as our Chief Operation Officer since May 2018. Mr. Tsimerman has over 15 years of experience as chief financial officer of public and private companies. He has served as chief financial officer of Insuline Medical Ltd. from June 2015 to May 2017, as chief financial officer of Mer-Group Broadband division (TASE: CMER) from 2005 to 2013 and as vice president of finance of Mer-Group Telecom Division from 2013 to 2014. Mr. Tsimerman received his Bachelor in business and MBA from The College of Management, Israel.
Shay Levav, Vice President, Regulatory and Quality Assurance and Clinical Applications
Mr. Shay Levav has served as our Vice President, Regulatory and Quality Assurance and Clinical Applications since September 2020. Before joining the Company, Mr. Levav has served as a member on the board of directors of Applied Spectral Imaging from December 2018 to September 2020 and as its quality and regulatory affairs manager since January 2015 to December 2018. He has also served as commercialization business manager of Carestream Health Inc. from 2012 to 2015, as its operations quality manager since 2001 to 2012, and service engineer since 2000 to 2001. Mr. Levav holds a B.A. from the Ruppin Academy Center, Israel.
Tlalit Bussi Tel-Tzure, Vice President, Business Development and Global Marketing
Ms. Tlalit Bussi Tel-Tzure has served as our Vice President, Business Development and Global Marketing, since December 2018. Ms. Bussi Tel-Tzure has more than 20 years of sales, business developments and marketing of medical device companies. She has served as vice president of marketing of DiA Imaging Analysis Ltd. form April 2017 to December 2018, and as vice president of sales and marketing of Medical Compression System Ltd. from September 2012 to August 2017. Ms. Bussi Tel-Tzure received her Bachelor of Science from the Hebrew University, Israel and her MBA from the Heriot-Watt University, United Kingdom.
Naum Muchnik, Vice President, Research, Development and Engineering
Mr. Naum Muchnik has served as our Vice President, Research, Development and Engineering since March 2018. Prior to that, Mr. Muchnik has served as operations and service manager of Medasense Biometrics Ltd. from August 2016 to March 2018 and as research and development mechanical team leader and project leader of GE Healthcare – Ultrasound. Mr. Muchnik received his M.Sc. in technology management from the Holon Technology Institute, Israel and Bachelor of Technology in mechanical engineering from the Ort Braude Academic College, Israel.
Doron Birger, Director
Mr. Doron Birger has served on our board of directors since August 2012. Mr. Birger has also served as chief executive officer of Doron Birger Management and Consulting, as the chairman of the board of directors of Nurami Medical Ltd. since April 2016, Sight Diagnostic Ltd. since June 2014, Onsight Medical Imaging Ltd. from June 2019 Intelicanna Ltd. (TASE: INTL) from April 2021 and Matricelf Ltd. from December 2020 and as a director of Vibrant Ltd. since December 2014, Hera Med Ltd. (ASX: HMD) since November 2019, Citrine Global (OTC: CTGL) since March 2020, Kadimastem Ltd. (TASE: KDST) since December 2020 and Netiv Ha’or, a subsidiary of the Israel Electric Corporation Ltd. since March 2020 and as chairman and director in a variety of non-profit organizations. Prior to that, Mr. Birger has served as member of the board of directors of MCS Medical Compression Systems (DBN) Ltd. (TASE:MDCL) from March 2015 to May 2018, Mekorot National Water Company Ltd. from November 2015 to November 2018, and chairman of the board of directors of Insulin Medical Ltd. (TASE: INSL) from March 2016 to August 2017, IOPtima Ltd. from June 2012 to June 2019, MST Medical Surgical Technologies Ltd. from August 2009 to June 2019, Highcon Ltd. from November 2014 to January 2018, Magisto Ltd. from September 2009 to July 2019, Real Imaging Ltd. from November 2018 to April 2019 and Medigus Ltd. (Nasdaq and TASE: MDGS) from May 2015 to September 2018. Mr. Birger received his B.A and M.A in economics from the Hebrew University, Israel.
88
Yang Huang, Director
Mr. Yang Huang has served on our board of directors since April 2020. Mr. Huang has 20 years of senior sales and marketing management experience in the field of medical devices. Mr. Huang has also served as operation directors of Virtus Inspire Ventures, a private equity fund, since July 2019 and as a corporate representative of IceCure (Shanghai) MedTech Co., Ltd. since July 2020, Prior to that, Mr. Huang has served as business unit director of Olympus (Beijing) Sales & Service Co., Ltd. from November 2016 to July 2019 and as business unit director of B. Braun MEDICAL (SHANGHAI) International Trading Co., Ltd. from January 2015 to November 2016. Mr. Huang has graduated from Cheung Kong Graduate School of Business, China and Zhejiang Medical University, China.
Sharon Levita, Director
Ms. Sharon Levita has served on our board of directors since September 2019. Ms. Levita also serves as business development and strategy leader of Medtronic. Prior to that, Ms. Levita has served as vice president operations and site leader of Mazor, as part of Medtronics, from December 2018 to January 2020 and as chief financial officer and vice president business operations of Mazor Robotics Ltd. from February 2008 to December 2018. Ms. Levita received her B.A in economics and accounting from the Haifa University, Israel and her M.A in business administration from the Bar-Ilan University, Israel. Ms. Levita is also a certificated public accountant in Israel.
Oded Tamir, Director
Mr. Oded Tamir has served on our board of directors since August 2013. Mr. Tamir has also served as chairman of the board of directors of Fertigo Medical Ltd. since November 2018 and as a member of the advisory board of Biodesign Israel, a medical entrepreneurship and innovation program, from June 2018. Prior to that, Mr. Tamir has served as chairman of Imedis AI Ltd. from November 2018 to September 2020 and LensFree Ltd. from June 2019 to September 2020, and as president and chief executive officer of RADLogics Inc. from June 2013 to December 2016. Mr. Tamir received his B.Sc. in economics and business management from the Technion – Israel Institute of Technology, Israel completed advanced accounting studies at the University of Haifa, Israel executive development management program at the Techions – Institute of Management, Israel and executive education and training program at the John F. Welch Leadership Development Center, New York.
Family Relationships
There are no family relationships between any members of our executive management and our directors.
Arrangements for Election of Directors and Members of Management
With the exception of our director, Yang Huang, who was appointed by Epoch Partner Investments Limited, one of our shareholders, there are no arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our executive management or our directors were selected (see “Related Party Transactions” for additional information).
Compensation
The following table sets forth information concerning the compensation of our named executive officers for the year ended December 31, 2020. The table does not include any amounts we paid to reimburse any of such persons for costs incurred in providing us with services during this period.
All amounts reported in the tables below reflect the cost to the Company, in thousands of U.S. Dollars, for the year ended December 31, 2020.
89
| Name and Principal Position |
Salary, Bonuses, Pension, Retirement and Other Similar Benefits |
Share Based Compensation |
Total | |||||||||
|
Eyal Shamir Chief Executive Officer |
$ | 370 | $ | 50 | $ | 420 | ||||||
|
Ronen Tsimerman Chief Financial Officer and Chief Operation Officer |
$ | 263 | $ | 35 | $ | 298 | ||||||
|
Elisabeth Sadka (1) Former VP Regulatory and Quality Assurance and Clinical Applications |
$ | 185 | $ | -20 | (2) | $ | 165 | |||||
|
Naum Muchnik VP Research, Development and Engineering |
$ | 215 | $ | 21 | $ | 236 | ||||||
|
Shay Levav (3) VP Regulatory and Quality Assurance and Clinical Applications |
$ | 70 | $ | 8 | $ | 78 | ||||||
|
Tlalit Bussi Tel-Tzure VP Buisness Development and Global Marketing |
$ | 232 | $ | 31 | $ | 263 | ||||||
| (1) | Ms. Sadka terminated her position as VP Regulatory and Quality Assurance and Clinical Applications of the Company in October 3, 2020. |
| (2) | Upon termination of her employment with the Company, Ms. Sadka’s unvested options were forfeited. |
| (3) | Mr. Levav commenced his position as VP Regulatory and Quality Assurance and Clinical Applications of the Company in September 2020. |
Employment Agreements with Executive Officers
We have entered into written employment agreements with each of our executive officers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we have entered into agreements with each executive officer and director pursuant to which we have agreed to indemnify each of them up to a certain amount and to the extent that these liabilities are not covered by directors’ and officers’ insurance.
Employment Agreement with Eyal Shamir as Chief Executive Officer
On August 7, 2016, we entered into an employment agreement with Mr. Shamir to act as our Chief Executive Officer, effective as of November 1, 2016. Mr. Shamir currently receives a monthly base salary of NIS 67,500 (approximately $20,633). He may also receive, at the sole discretion of the board of directors and the compensation committee, an additional cash performance-based bonuses at the end of each year of up to three months’ base salary and a discretionary equity award equal to additional two months’ salary. The employment agreement may be terminated by the Company or by Mr. Shamir at any time and for any reason upon a 90-day prior written notice.
Employment Agreement with Ronen Tsimerman as Chief Financial Officer and Chief Operation Officer
On March 29, 2017, we entered into an employment agreement with Mr. Tsimerman to act as our Chief Financial Officer, effective as of May 1, 2017, and as our Chief Operation Officer as of May 21, 2018. Mr. Tsimerman currently receives a monthly base salary of NIS 45,000 (approximately $13,755). He may also receive, at the sole discretion of the board of directors and the compensation committee, an additional cash performance-based bonuses at the end of each year of up to three months’ base salary and an additional discretionary equity award equal to an additional one month salary. The employment agreement may be terminated by the Company or by Mr. Tsimerman at any time and for any reason upon a 90-day prior written notice.
Employment Agreement with Naum Mutchnik as VP Research, Development and Engineering
On February 20, 2018, we entered into an employment agreement with Mr. Muchnik to act as our VP Research and Development, effective as of March 21, 2018. Mr. Muchnik currently receives a monthly base salary of NIS 40,000 (approximately $12,227). He may also receive, at the sole discretion of the board of directors and the compensation committee, an additional cash performance-based bonuses at the end of each year of up to three months’ base salary and an additional discretionary equity award equal to additional one month salary. The employment agreement may be terminated by the Company or by Mr. Muchnik at any time and for any reason upon a 90-day prior written notice.
90
Employment Contract with Mr. Shay Levav as VP Regulatory and Quality Assurance and Clinical Applications
On July 12, 2020, we entered into an employment agreement with Mr. Levav to act as our VP Regulatory and Quality Assurance and Clinical Applications, effective as of September 6, 2020. Mr. Levav currently receives a monthly base salary of NIS 40,000 (approximately $12,227). He may also receive, at the sole discretion of the board of directors and the compensation committee, an additional cash performance-based bonuses at the end of each year up to three months’ base salary and an additional discretionary equity award equal to an additional one month salary. The employment agreement may be terminated by the Company or by Mr. Levav at any time and for any reason upon a 60-day prior written notice .
Employment Contract with Ms. Tlalit Bussi Tel-Tzur as VP Business Development and Global Marketing
On February 13, 2019, we entered into an employment agreement with Ms. Tel-Tzur to act as our VP Business Development and Global Marketing, effective as of February 13, 2019. Ms. Tel-Tzur currently receives a monthly base salary of NIS 42,000 (approximately $12,838). She may also receive, at the sole discretion of the board of directors and compensation committee, an additional cash performance-based bonuses at the end of each year of up to three months’ base salary and an additional discretionary equity award equal to an additional one month salary. The employment agreement may be terminated by the Company or by Ms. Tel-Tzur at any time and for any reason upon a 90-day prior written notice.
For a description of the terms of our options and option plans (see “Management—Share Incentive Plan” below).
Directors’ Service Contracts
Other than with respect to our directors that are also executive officers, we do not have written agreements with any director providing for benefits upon the termination of his employment with our company.
Differences between the Companies Law and Nasdaq Requirements
Companies incorporated under the laws of the State of Israel whose shares are publicly traded, including companies with shares listed on Nasdaq, are considered public companies under Israeli law and are required to comply with various corporate governance requirements under Israeli law relating to such matters as the composition and responsibilities of the audit committee and the compensation committee (subject to certain exceptions that we intend to utilize), and a requirement to have an internal auditor. These requirements are in addition to the corporate governance requirements imposed by the rules of The Nasdaq Stock Market and other applicable provisions of U.S. securities laws to which we will become subject (as a foreign private issuer) upon the closing of this offering and the listing of our ordinary shares on Nasdaq. Under the Nasdaq Stock Market Rules, a foreign private issuer may generally follow its home country rules of corporate governance in lieu of the comparable requirements of the Nasdaq Rules, except for certain matters including the composition and responsibilities of the audit committee.
In accordance with Israeli law and practice and subject to the exemption set forth in Rule 5615 of the Nasdaq Stock Market rules, we have elected to follow the provisions of the Companies Law, rather than the Nasdaq Stock Market rules, with respect to the following requirements:
| ● | Distribution of periodic reports to shareholders; proxy solicitation. As opposed to the Nasdaq Stock Market rules, which require listed issuers to make such reports available to shareholders in one of a number of specific manners, Israeli law does not require us to distribute periodic reports directly to shareholders, and the generally accepted business practice in Israel is not to distribute such reports to shareholders but to make such reports available through a public website. In addition to making such reports available on a public website, we currently make our audited consolidated financial statements available to our shareholders at our offices and will only mail such reports to shareholders upon request. As a foreign private issuer, we are generally exempt from the SEC’s proxy solicitation rules. |
| ● | Quorum. While the Nasdaq Stock Market rules require that the quorum for purposes of any meeting of the holders of a listed company’s common voting stock, as specified in the company’s bylaws, be no less than 33 1/3% of the company’s outstanding common voting stock, under Israeli law, a company is entitled to determine in its articles of association the number of shareholders and percentage of holdings required for a quorum at a shareholders meeting. Our amended and restated articles of association provide that a quorum of two or more shareholders holding at least two (2) shareholders, in person or by proxy, holding at least 25% of the voting rights. in person or by proxy is required for commencement of business at a general meeting. However, the quorum set forth in our amended and restated articles of association with respect to an adjourned meeting consists of at least one shareholders present in person or by proxy. |
| ● | Nomination of our directors. With the exception of directors elected by our board of directors and external directors, our directors are elected by an annual or special meeting of our shareholders (i) to hold office until the next annual meeting following his or her election or (ii) for three-year term, as described below under “Management—Board Practices—External Directors.” The nominations for directors, which are presented to our shareholders by our board of directors, are generally made by the board of directors itself, in accordance with the provisions of our amended and restated articles of association and the Companies Law. Nominations need not be made by a nominating committee of our board of directors consisting solely of independent directors, as required under the Nasdaq Stock Market rules. |
91
| ● | Compensation of officers. Israeli law and our amended and restated articles of association do not require that the independent members of our board of directors (or a compensation committee composed solely of independent members of our board of directors) determine an executive officer’s compensation, as is generally required under the Nasdaq Stock Market rules with respect to the chief executive officer and all other executive officers. Instead, compensation of executive officers is determined and approved by our compensation committee and our board of directors, and in certain circumstances by our shareholders, either in consistency with our office holder compensation policy or, in special circumstances in deviation therefrom, taking into account certain considerations stated in the Companies Law (see “Management—Board Practices—Approval of Related Party Transactions under Israeli Law” for additional information). |
| ● | Independent directors. Israeli law does not require that a majority of the directors serving on our board of directors be “independent,” as defined under Nasdaq Listing Rule 5605(a)(2), and rather requires we have at least two external directors who meet the requirements of the Companies Law, as described above under “Management—Board Practices—External Directors.” We are required, however, to ensure that all members of our audit committee are “independent” under the applicable Nasdaq and SEC criteria for independence (as we cannot exempt ourselves from compliance with that SEC independence requirement, despite our status as a foreign private issuer), and we must also ensure that a majority of the members of our audit committee are “independent directors” as defined in the Companies Law. Furthermore, Israeli law does not require, nor do our independent directors conduct, regularly scheduled meetings at which only they are present, which the Nasdaq Stock Market rules otherwise require. |
| ● | Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Companies Law, rather than seeking approval for corporation actions in accordance with Nasdaq Listing Rule 5635. In particular, under this Nasdaq Stock Market rule, shareholder approval is generally required for: (i) an acquisition of shares/assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest in the target company or the consideration to be received; (ii) the issuance of shares leading to a change of control; (iii) adoption/amendment of equity compensation arrangements (although under the provisions of the Companies Law there is no requirement for shareholder approval for the adoption/amendment of the equity compensation plan); and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Companies Law, shareholder approval is required for, among other things: (i) transactions with directors concerning the terms of their service or indemnification, exemption and insurance for their service (or for any other position that they may hold at a company), for which approvals of the compensation committee, board of directors and shareholders are all required, (ii) extraordinary transactions with controlling shareholders of publicly held companies, which require the special approval, and (iii) terms of employment or other engagement of the controlling shareholder of us or such controlling shareholder’s relative, which require special approval. In addition, under the Companies Law, a merger requires approval of the shareholders of each of the merging companies. |
| ● | Approval of Related Party Transactions. All related party transactions are approved in accordance with the requirements and procedures for approval of interested party acts and transaction as set forth in the Companies Law, which requires the approval of the audit committee, or the compensation committee, as the case may be, the board of directors and shareholders, as may be applicable, for specified transactions, rather than approval by the audit committee or other independent body of our board of directors as required under the Nasdaq Stock Market rules (see “Management—Board Practices—Approval of Related Party Transactions under Israeli Law” for additional information). |
| ● | Annual Shareholders Meeting. As opposed to the Nasdaq Stock Market Rule 5620(a), which mandates that a listed company hold its annual shareholders meeting within one year of the company’s fiscal year-end, we are required, under the Companies Law, to hold an annual shareholders meeting each calendar year and within 15 months of the last annual shareholders meeting. |
Board Practices
Introduction
Our board of directors presently consists of six members, including two external directors required to be appointed under the Companies Law. We believe that Ms. Sharon Levita, Mr. Oded Tamir and Mr. Doron Birger are “independent” for purposes of the Nasdaq Stock Market rules. Our amended and restated articles of association provide that the number of board of directors’ members (including external directors) shall be set by the general meeting of the shareholders provided that it will consist of not less than five (5) and not more than eleven (11). Pursuant to the Companies Law, the management of our business is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are appointed by our Chief Executive Officer. Their terms of employment are subject to the approval of the compensation committee and of the board of directors, and are subject to the terms of any applicable employment agreements that we may enter into with them.
92
Each director, except external directors, will hold office until the next annual general meeting of our shareholders following his or her appointment, or until he or she resigns or unless he or she is removed by a majority vote of our shareholders at a general meeting of our shareholders or upon the occurrence of certain events, in accordance with the Companies Law and our amended and restated articles of association.
In addition, under certain circumstances, our amended and restated articles of association allow our board of directors to appoint directors to fill vacancies on our board of directors or in addition to the acting directors (subject to the limitation on the number of directors), until the next annual general meeting or special general meeting in which directors may be appointed or terminated. External directors may be elected for up to two additional three-year terms after their initial three-year term under the circumstances described below, with certain exceptions as described in “External Directors” below. External directors may be removed from office only under the limited circumstances set forth in the Companies Law (see “Management—Board Practices—External Directors” below).
Under the Companies Law, any shareholder holding at least one percent of our outstanding voting power may nominate a director. However, any such shareholder may make such a nomination only if a notice of such shareholder’s intent to make such nomination has been given to our board of directors. Any such notice must include certain information, including the consent of the proposed director nominee to serve as our director if elected, and a declaration that the nominee signed declaring that he or she possesses the requisite skills and has the availability to carry out his or her duties. Additionally, the nominee must provide details of such skills, and demonstrate an absence of any limitation under the Companies Law that may prevent his or her election, and affirm that all of the required election-information is provided to us, pursuant to the Companies Law.
Under the Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial expertise. In determining the number of directors required to have such expertise, our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the minimum number of directors of our company who are required to have accounting and financial expertise is one.
The board of directors must elect one director to serve as the chairman of the board of directors to preside at the meetings of the board of directors, and may also remove that director as chairman. Pursuant to the Companies Law, neither the chief executive officer nor any of his or her relatives is permitted to serve as the chairman of the board of directors, and a company may not vest the chairman or any of his or her relatives with the chief executive officer’s authorities. In addition, a person who reports, directly or indirectly, to the chief executive officer may not serve as the chairman of the board of directors; the chairman may not be vested with authorities of a person who reports, directly or indirectly, to the chief executive officer; and the chairman may not serve in any other position in the company or a controlled company, but he or she may serve as a director or chairman of a controlled company. However, the Companies Law permits a company’s shareholders to determine, for a period not exceeding three years from each such determination, that the chairman or his or her relative may serve as chief executive officer or be vested with the chief executive officer’s authorities, and that the chief executive officer or his or her relative may serve as chairman or be vested with the chairman’s authorities. Such determination of a company’s shareholders requires either: (1) the approval of at least a majority of the shares of those shareholders present and voting on the matter (other than controlling shareholders and those having a personal interest in the determination) (shares held by abstaining shareholders shall not be considered); or (2) that the total number of shares opposing such determination does not exceed 2% of the total voting power in the company. Currently, we have a separate chairman and chief executive officer.
The board of directors may, subject to the provisions of the Companies Law, delegate any or all of its powers to committees of the board, and it may, from time to time, revoke such delegation or alter the composition of any such committees, subject to certain limitations. Unless otherwise expressly provided by the board of directors, the committees shall not be empowered to further delegate such powers. The composition and duties of our audit committee, financial statement examination committee and compensation committee are described below.
The board of directors oversees how management monitors compliance with our risk management policies and procedures, and reviews the adequacy of the risk management framework in relation to the risks faced by us. The board of directors is assisted in its oversight role by an internal auditor. The internal auditor undertakes both regular and ad hoc reviews of risk management controls and procedures, the results of which are reported to our audit committee.
93
External Directors
Under the Companies Law, an Israeli company whose shares have been offered to the public or whose shares are listed for trading on a stock exchange in or outside of Israel is required to appoint at least two external directors to serve on its board of directors. External directors must meet stringent standards of independence. As of the date hereof, our external directors are Ms. Sharon Levita and Mr. Oded Tamir.
According to regulations promulgated under the Companies law, at least one of the external directors is required to have “financial and accounting expertise,” unless another member of the audit committee, who is an independent director under the Nasdaq Stock Market rules, has “financial and accounting expertise,” and the other external director or directors are required to have “professional expertise.” An external director may not be appointed to an additional term unless: (1) such director has “accounting and financial expertise;” or (2) he or she has “professional expertise,” and on the date of appointment for another term there is another external director who has “accounting and financial expertise” and the number of “accounting and financial experts” on the board of directors is at least equal to the minimum number determined appropriate by the board of directors. We have determined that both Ms. Sharon Levita and Mr. Oded Tamir have accounting and financial expertise.
A director with accounting and financial expertise is a director who, due to his or her education, experience and skills, possesses a high degree of proficiency in, and an understanding of, business – accounting matters and financial statements, such that he or she is able to understand the financial statements of the company in depth and initiate a discussion about the manner in which financial data is presented. A director is deemed to have “professional expertise” if he or she holds an academic degree in certain fields or has at least five years of experience in one of the said fields or in certain senior positions.
External directors are elected by a majority vote at a shareholders’ meeting, as long as either:
| ● | at least a majority of the shares held by shareholders who are not controlling shareholders and do not have personal interest in the appointment (excluding a personal interest that did not result from the shareholder’s relationship with the controlling shareholder) have voted in favor of the proposal (shares held by abstaining shareholders shall not be considered); or |
| ● | the total number of shares voted against the election of the external director, does not exceed 2% of the aggregate voting rights of the company. |
The term “control” is defined in the Companies Law as the ability to direct the activities of the company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder “holds” (within the meaning of the Companies Law) 50% or more of the voting rights in a company or has the right to appoint 50% or more of the directors of the company or its general manager. With respect to certain matters (for example: interested party transactions), a controlling shareholder is deemed to include a shareholder that holds 25% or more of the voting rights in a public company if no other shareholder holds more than 50% of the voting rights in the company, but excludes a shareholder whose power derives solely from his or her position as a director of the company or from any other position with the company.
The Companies Law provides for an initial three-year term for an external director. Thereafter, an external director may be reelected by shareholders to serve in that capacity for up to two additional three-year terms, provided that:
| (1) | his or her service for each such additional term is recommended by one or more shareholders holding at least one percent of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds two percent of the aggregate voting rights in the company and subject to additional restrictions set forth in the Companies Law with respect to the affiliation of the external director nominee as described below; |
| (2) | his or her service for each such additional term is recommended by the board of directors and is approved at a shareholders meeting by the same disinterested majority required for the initial election of an external director (as described above); or |
94
| (3) | the external director offered his or her service for each such additional term and was approved in accordance with the provisions of section (1) above. |
The term of office for external directors for Israeli companies traded on certain foreign stock exchanges, including the Nasdaq Stock Market, may be extended indefinitely in increments of additional three-year terms, in each case provided that the audit committee and the board of directors of the company confirm that, in light of the external director’s expertise and special contribution to the work of the board of directors and its committees, the reelection for such additional period(s) is beneficial to the company, and provided that the external director is reelected subject to the same shareholder vote requirements as if elected for the first time (as described above). Prior to the approval of the reelection of the external director at a general shareholders meeting, the company’s shareholders must be informed of the term previously served by him or her and of the reasons why the board of directors and audit committee recommended the extension of his or her term.
The Companies Law provides that a person is not qualified to serve as an external director if (i) the person is a relative of a controlling shareholder of the company, or (ii) if that person or his or her relative, partner, employer, another person to whom he or she was directly or indirectly subordinate, or any entity under the person’s control, has or had, during the two years preceding the date of appointment as an external director: (a) any affiliation or other disqualifying relationship with the company, with any person or entity controlling the company or a relative of such person, or with any entity controlled by or under common control with the company; or (b) in the case of a company with no shareholder holding 25% or more of its voting rights, had at the date of appointment as an external director, any affiliation or other disqualifying relationship with a person then serving as chairman of the board or chief executive officer, with a holder of 5% or more of the issued share capital or voting power in the company or with the most senior financial officer.
The term “relative” is defined under the Companies Law as a spouse, sibling, parent, grandparent or descendant; spouse’s sibling, parent or descendant; and the spouse of each of the foregoing persons.
Under the Companies Law, the term “affiliation” and the similar types of disqualifying relationships include (subject to certain exceptions):
| ● | an employment relationship; |
| ● | a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships); |
| ● | control; and |
| ● | service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director was appointed as a director of the private company in order to serve as an external director following the initial public offering. |
The term “office holder” is defined under the Companies Law as a general manager, chief business manager, deputy general manager, vice general manager, any other person assuming the responsibilities of any of these positions regardless of that person’s title, a director and any other manager directly subordinate to the general manager.
In addition, no person may serve as an external director if that person’s position or professional or other activities create, or may create, a conflict of interest with that person’s responsibilities as a director or otherwise interfere with that person’s ability to serve as a director or if the person is an employee of the Israel Securities Authority or of an Israeli stock exchange. A person may furthermore not continue to serve as an external director if he or she received direct or indirect compensation from the company including amounts paid pursuant to indemnification and/or exculpation contracts or commitments and insurance coverage, other than for his or her service as an external director as permitted by the Companies Law and the regulations promulgated thereunder.
95
Following the termination of an external director’s service on a board of directors, such former external director and his or her spouse and children may not be provided a direct or indirect benefit by the company, its controlling shareholder or any entity under its controlling shareholder’s control. This includes engagement as an office holder or director of the company or a company controlled by its controlling shareholder or employment by, or provision of services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by the former external director. This restriction extends for a period of two years with regard to the former external director and his or her spouse or child and for one year with respect to other relatives of the former external director.
External directors may be removed only by a special general meeting of shareholders called by the board of directors after the board has determined the occurrence of circumstances allow such dismissal, at the same special majority of shareholders required for their election or by a court, and in both cases only if the external directors cease to meet the statutory qualifications for their appointment or if they violate their duty of loyalty to our company. In the event of a vacancy created by an external director which causes the company to have fewer than two external directors, the board of directors is required under the Companies Law to call a shareholders meeting as soon as possible to appoint such number of new external directors in order that the company thereafter has two external directors.
External directors may be compensated only in accordance with regulations adopted under the Companies Law.
If at the time at which an external director is appointed all members of the board of directors who are not controlling shareholders or relatives of controlling shareholders of the company are of the same gender, the external director to be appointed must be of the other gender. A director of a company may not be appointed as an external director of another company if at the same time a director of such other company is acting as an external director of the first company.
under regulations promulgated pursuant to the Companies Law, a company with no controlling shareholder whose shares are listed for trading on specified exchanges outside of Israel, including the Nasdaq Capital Market, may adopt exemptions from various corporate governance requirements of the Companies Law, so long as such company satisfies the requirements of applicable foreign country laws and regulations, including applicable stock exchange rules, that apply to companies organized in that country and relating to the appointment of independent directors and the composition of audit and compensation committees. Such exemptions include an exemption from the requirement to appoint external directors and the requirement that an external director be a member of certain committees, as well as exemption from limitations on directors’ compensation. As of the Date hereof, the Company have a controlling shareholder and therefore cannot use such exemptions.
Independent Directors Under the Companies Law
An “independent director” is either an external director or a director who meets the same non-affiliation criteria as an external director (except for (i) the requirement that the director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed outside of Israel) and (ii) the requirement for accounting and financial expertise or professional qualifications), as determined by the audit committee, and who has not served as a director of the company for more than nine consecutive years. For these purposes, ceasing to serve as a director for a period of two years or less would not be deemed to sever the consecutive nature of such director’s service.
Regulations promulgated pursuant to the Companies Law provide that a director in a public company whose shares are listed for trading on specified exchanges outside of Israel, including the Nasdaq Capital Market, who qualifies as an independent director under the relevant non-Israeli rules and who meets certain non-affiliation criteria, which are less stringent than those applicable to independent directors as set forth above, would be deemed an “independent” director pursuant to the Companies Law provided: (i) he or she has not served as a director for more than nine consecutive years; (ii) he or she has been approved as such by the audit committee; and (iii) his or her remuneration shall be in accordance with the Companies Law and the regulations promulgated thereunder. For these purposes, ceasing to serve as a director for a period of two years or less would not be deemed to sever the consecutive nature of such director’s service.
Furthermore, pursuant to these regulations, such company may reappoint a person as an independent director for additional terms, beyond nine years, which do not exceed three years each, if each of the audit committee and the board of directors determine, in that order, that in light of the independent director’s expertise and special contribution to the board of directors and its committees, the reappointment for an additional term is in the company’s best interest.
96
Alternate Directors
Our amended and restated articles of association provide, as allowed by the Companies Law, that any director may, subject to the conditions set thereto including approval of the nominee by our board of directors, appoint a person as an alternate to act in his place, to remove the alternate and appoint another in his place and to appoint an alternate in place of an alternate whose office is vacated for any reason whatsoever. Under the Companies Law, a person who is not qualified to be appointed as a director, a person who is already serving as a director or a person who is already serving as an alternate director for another director, may not be appointed as an alternate director. Nevertheless, a director who is already serving as a director may be appointed as an alternate director for a member of a committee of the board of directors so long as he or she is not already serving as a member of such committee, and if the alternate director is to replace an external director, he or she is required to be an external director and to have either “financial and accounting expertise” or “professional expertise,” depending on the qualifications of the external director he or she is replacing. A person who does not have the requisite “financial and accounting experience” or the “professional expertise,” depending on the qualifications of the external director he or she is replacing, may not be appointed as an alternate director for an external director. A person who is not qualified to be appointed as an independent director, pursuant to the Companies Law, may not be appointed as an alternate director of an independent director qualified as such under the Companies Law. Unless the appointing director limits the time or scope of the appointment, the appointment is effective for all purposes until the appointing director ceases to be a director or terminates the appointment.
Committees of the Board of Directors
Our board of directors has established three standing committees, the audit committee, the compensation committee and the Financial Statement Examination Committee.
Audit Committee
Under the Companies Law, we are required to appoint an audit committee. The audit committee must be comprised of at least three directors, including all of the external directors (one of whom must serve as chair of the committee). The audit committee may not include the chairman of the board; a controlling shareholder of the company or a relative of a controlling shareholder; a director employed by or providing services on a regular basis to the company, to a controlling shareholder or to an entity controlled by a controlling shareholder; or a director who derives most of his or her income from a controlling shareholder.
In addition, a majority of the members of the audit committee of a publicly traded company must be independent directors under the Companies Law. Our audit committee is comprised of Ms. Sharon Levita, Mr. Oded Tamir and Mr. Doron Birger.
Under the Companies Law, our audit committee is responsible for:
| (i) | determining whether there are deficiencies in the business management practices of our company, and making recommendations to the board of directors to improve such practices; | |
| (ii) | determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest and whether such transaction is extraordinary or material under Companies Law) and establishing the approval process for certain transactions with a controlling shareholder or in which a controlling shareholder has a personal interest (see “Management—Board Practices—Approval of Related Party Transactions under Israeli law”); | |
| (iii) | determining the approval process for transactions that are “non-negligible” (i.e., transactions with a controlling shareholder that are classified by the audit committee as non-negligible, even though they are not deemed extraordinary transactions), as well as determining which types of transactions would require the approval of the audit committee, optionally based on criteria which may be determined annually in advance by the audit committee; | |
| (iv) | examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities; |
97
| (v) | examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment of our auditor; | |
| (vi) | establishing procedures for the handling of employees’ complaints as to deficiencies in the management of our business and the protection to be provided to such employees; and | |
| (vii) | where the board of directors approves the working plan of the internal auditor, examining such working plan before its submission to the board of directors and proposing amendments thereto. |
Our audit committee may not conduct any discussions or approve any actions requiring its approval (see “Management—Board Practices—Approval of Related Party Transactions under Israeli law”), unless at the time of the approval a majority of the committee’s members are present, which majority consists of independent directors under the Companies Law, including at least one external director.
Our board of directors intends to adopt an audit committee charter to be effective upon the listing of the Ordinary Shares on the Nasdaq Capital Market setting forth, among others, the responsibilities of the audit committee consistent with the rules of the SEC and Nasdaq Listing Rules (in addition to the requirements for such committee under the Companies Law), including, among others, the following:
| ● | oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors in accordance with Israeli law; |
| ● | recommending the engagement or termination of the person filling the office of our internal auditor, reviewing the services provided by our internal auditor and reviewing effectiveness of our system of internal control over financial reporting; |
| ● | recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors; and |
| ● | reviewing and monitoring, if applicable, legal matters with significant impact, finding of regulatory authorities’ findings, receive reports regarding irregularities and legal compliance, acting according to “whistleblower policy” and recommend to our board of directors if so required. |
Nasdaq Stock Market Requirements for Audit Committee
Under the Nasdaq Stock Market rules, we are required to maintain an audit committee consisting of at least three members, all of whom are independent and are financially literate and one of whom has accounting or related financial management expertise.
As noted above, the members of our audit committee include Ms. Sharon Levita and Mr. Oded Tamir who are external directors, and Mr. Doron Birger who is an independent director, each of whom is “independent,” as such term is defined in under Nasdaq Stock Market rules. Ms. Levita serves as the chairman of our audit committee. All members of our audit committee meet the requirements for financial literacy under the Nasdaq Stock Market rules. Our board of directors has determined that each member of our audit committee is an audit committee financial expert as defined by the SEC rules and has the requisite financial experience as defined by the Nasdaq Stock Market rules.
Financial Statement Examination Committee
Under the Companies Law, the board of directors of a public company in Israel must appoint a financial statement examination committee, which consists of members with accounting and financial expertise or the ability to read and understand financial statements. Our financial statement examination committee is comprised of Ms. Sharon Levita, Mr. Oded Tamir and Mr. Doron Birger. The function of a financial statements examination committee is to discuss and provide recommendations to its board of directors (including the report of any deficiency found) with respect to the following issues: (1) estimations and assessments made in connection with the preparation of financial statements; (2) internal controls related to the financial statements; (3) completeness and propriety of the disclosure in the financial statements; (4) the accounting policies adopted and the accounting treatments implemented in material matters of the company; and (5) value evaluations, including the assumptions and assessments on which evaluations are based and the supporting data in the financial statements. Our independent registered public accounting firm and our internal auditor are invited to attend all meetings of our financial statements examination committee.
98
Compensation Committee
Under the Companies Law, the board of directors of any public company must establish a compensation committee. The compensation committee must be comprised of at least three directors, including all of the external directors, who must constitute a majority of the members of the compensation committee. Each compensation committee member that is not an external director must be a director whose compensation does not exceed an amount that may be paid to an external director. The compensation committee is subject to the same Companies Law restrictions as the audit committee as to: (a) who may not be a member of the committee; and (b) who may not be present during committee deliberations as described above.
Our compensation committee is acting pursuant to a written charter, and consists of Ms. Sharon Levita, Mr. Oded Tamir and Mr. Doron Birger. Our compensation committee complies with the provisions of the Companies Law, the regulations promulgated thereunder, and our amended and restated articles of association, on all aspects referring to its independence, authorities and practice. Our compensation committee follows home country practice as opposed to complying with the compensation committee membership and charter requirements prescribed under the Nasdaq Stock Market rules.
Our compensation committee reviews and recommends to our board of directors: with respect to our executive officers’ and directors’: (1) annual base compensation (2) annual incentive bonus, including the specific goals and amounts; (3) equity compensation; (4) employment agreements, severance arrangements, and change in control agreements and provisions; (5) retirement grants and/or retirement bonuses; and (6) any other benefits, compensation, compensation policies or arrangements.
The duties of the compensation committee include the recommendation to the company’s board of directors of a policy regarding the terms of engagement of office holders, to which we refer as a compensation policy. Such policy must be adopted by the company’s board of directors, after considering the recommendations of the compensation committee. The compensation policy is then brought for approval by our shareholders, which requires a special majority (see “Management—Board Practices—Approval of Related Party Transactions under Israeli law”). Under the Companies Law, the board of directors may adopt the compensation policy if it is not approved by the shareholders, provided that after the shareholders oppose the approval of such policy, the compensation committee and the board of directors revisit the matter and determine that adopting the compensation policy would be in the best interests of the company. Our compensation policy was approved by our shareholders on April 2, 2020.
The compensation policy must serve as the basis for decisions concerning the financial terms of employment or engagement of executive officers and directors, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The compensation policy must relate to certain factors, including advancement of the company’s objectives, the company’s business and its long-term strategy, and creation of appropriate incentives for executives. It must also consider, among other things, the company’s risk management, size and the nature of its operations. The compensation policy must furthermore consider the following additional factors:
| ● | the education, skills, expertise and accomplishments of the relevant director or executive; |
| ● | the director’s or executive’s roles and responsibilities and prior compensation agreements with him or her; |
| ● | the relationship between the cost of the terms of service of an office holder and the average median compensation of the other employees of the company (including those employed through manpower companies), including the impact of disparities in salary upon work relationships in the company; |
| ● | the possibility of reducing variable compensation at the discretion of the board of directors; and the possibility of setting a limit on the exercise value of non-cash variable compensation; and |
| ● | as to severance compensation, the period of service of the director or executive, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company. |
99
The compensation policy must also include the following principles:
| ● | with the exception of office holders who report directly to the chief executive officer, the link between variable compensation and long-term performance and measurable criteria; |
| ● | the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation at the time of its grant; |
| ● | the conditions under which a director or executive would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements; |
| ● | the minimum holding or vesting period for variable, equity-based compensation; and |
| ● | maximum limits for severance compensation. |
The compensation policy must also consider appropriate incentives from a long-term perspective.
The compensation committee is responsible for: (1) recommending the compensation policy to a company’s board of directors for its approval (and subsequent approval by the shareholders); and (2) duties related to the compensation policy and to the compensation of a company’s office holders, including:
| ● | recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years); |
| ● | recommending to the board of directors periodic updates to the compensation policy; |
| ● | assessing implementation of the compensation policy; |
| ● | determining whether the terms of compensation of certain office holders of the company need not be brought to approval of the shareholders; and |
| ● | determining whether to approve the terms of compensation of office holders that require the committee’s approval. |
Our compensation policy is designed to promote our long-term goals, work plan and policy, retain, motivate and incentivize our directors and executive officers, while considering the risks that our activities involve, our size, the nature and scope of our activities and the contribution of an officer to the achievement of our goals and maximization of profits, and align the interests of our directors and executive officers with our long-term performance. To that end, a portion of an executive officer compensation package is targeted to reflect our short and long-term goals, as well as the executive officer’s individual performance. On the other hand, our compensation policy includes measures designed to reduce the executive officer’s incentives to take excessive risks that may harm us in the long-term, such as limits on the value of cash bonuses and equity-based compensation, limitations on the ratio between the variable and the total compensation of an executive officer and minimum vesting periods for equity-based compensation.
Our compensation policy also addresses our executive officer’s individual characteristics (such as his or her respective position, education, scope of responsibilities and contribution to the attainment of our goals) as the basis for compensation variation among our executive officers, and considers the internal ratios between compensation of our executive officers and directors and other employees. Pursuant to our compensation policy, the compensation that may be granted to an executive officer may include: base salary, annual bonuses, equity-based compensation, benefits and retirement and termination of service arrangements. All cash bonuses (including special bonuses, one-time bonus and conservation bonus) are limited to a maximum amount linked to the executive officer’s base salary. In addition, our compensation policy provides for maximum permitted ratios between the total variable (cash bonuses and equity based compensation) and non-variable (base salary) compensation components, in accordance with an officer’s respective position with the company.
100
An annual cash bonus may be awarded to executive officers upon the attainment of pre-set periodic objectives and individual targets. The annual cash bonus that may be granted to executive officers other than our chairman or Chief Executive Officer may be based entirely on a discretionary evaluation. Our Chief Executive Officer will be entitled to recommend performance objectives to such executive officers, and such performance objectives will be approved by our compensation committee (and, if required by law, by our board of directors).
The performance measurable objectives of our Chief Executive Officer will be determined annually by our compensation committee and board of directors. A less significant portion of Chief Executive Officer’s annual cash bonus may be based on a discretionary evaluation of the Chief Executive Officer’s respective overall performance by the compensation committee and the board of directors based on quantitative and qualitative criteria.
The equity-based compensation under our compensation policy for our executive officers (including members of our board of directors) is designed in a manner consistent with the underlying objectives in determining the base salary and the annual cash bonus, with its main objectives being to enhance the alignment between the executive officers’ interests with our long-term interests and those of our shareholders and to strengthen the retention and the motivation of executive officers in the long term. Our compensation policy provides for executive officer compensation in the form of share options in accordance with our share incentive plan then in place. Share options granted to executive officers shall be subject to vesting periods in order to promote long-term retention of the awarded executive officers. The equity-based compensation shall be granted from time to time and be individually determined and awarded according to the performance, educational background, prior business experience, qualifications, role and the personal responsibilities of the executive officer.
In addition, our compensation policy contains compensation recovery provisions which allows us under certain conditions to recover bonuses paid in excess, enables our Chief Executive Officer to approve an immaterial change in the terms of employment of an executive officer (provided that the changes of the terms of employment are in accordance our compensation policy) and allows us to exculpate, indemnify and insure our executive officers and directors subject to certain limitations set forth thereto.
Our compensation policy also provides for compensation to the members of our board of directors either: (i) in accordance with the amounts provided in the Companies Regulations (Rules Regarding the Compensation and Expenses of an External Director) of 2000, as amended by the Companies Regulations (Relief for Public Companies Traded in Stock Exchange Outside of Israel) of 2000, as such regulations may be amended from time to time; or (ii) in accordance with the amounts determined in our compensation policy.
Internal Auditor
Under the Companies Law, the board of directors of an Israeli public company must appoint an internal auditor nominated by the audit committee. Our internal auditor is Mr. Doron Cohen (Fahn Kanne Control Management Ltd, Grant Thornton). The role of the internal auditor is to examine, among other things, whether a company’s actions comply with the law and proper business procedure. The audit committee is required to oversee the activities, and to assess the performance of the internal auditor as well as to review the internal auditor’s work plan. An internal auditor may not be an interested party or office holder, or a relative of any interested party or office holder, and may not be a member of the company’s independent accounting firm or its representative. The Companies Law defines an interested party as a holder of 5% or more of the outstanding shares or voting rights of a company, any person or entity that has the right to appoint at least one director or the general manager of the company or any person who serves as a director or as the general manager of a company. Our internal auditor is not an interested party in the Company, and not our employee.
Remuneration of Directors
Under the Companies Law, remuneration of directors is subject to the approval of the compensation committee, thereafter by the board of directors and thereafter, unless exempted under the regulations promulgated under the Companies Law, by the general meeting of the shareholders. In case the remuneration of the directors is in accordance with regulations applicable to remuneration of the external directors then such remuneration shall be exempt from the approval of the general meeting. Where the director is also a controlling shareholder, the requirements for approval of transactions with controlling shareholders apply.
101
Fiduciary Duties of Office Holders
The Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company.
The duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care of an office holder includes a duty to use reasonable means to obtain:
| ● | information on the advisability of a given action brought for his approval or performed by him by virtue of his position; and |
| ● | all other important information pertaining to these actions. |
The duty of loyalty of an office holder requires an office holder to act in good faith and for the benefit of the company, and includes a duty to:
| ● | refrain from any conflict of interest between the performance of his duties in the company and his performance of his other duties or personal affairs; |
| ● | refrain from any action that is competitive with the company’s business; |
| ● | refrain from exploiting any business opportunity of the company to receive a personal gain for himself or others; and |
| ● | disclose to the company any information or documents relating to the company’s affairs which the office holder has received due to his position as an office holder. |
Insurance
Under the Companies Law, a company may obtain insurance for any of its office holders against the following liabilities incurred due to acts he or she performed as an office holder, if and to the extent provided for in the company’s articles of association:
| ● | breach of his or her duty of care to the company or to another person, to the extent such a breach arises out of the negligent conduct of the office holder; |
| ● | a breach of his or her duty of loyalty to the company, provided that the office holder acted in good faith and had reasonable cause to assume that his or her act would not prejudice the company’s interests; and |
| ● | a financial liability imposed upon him or her in favor of another person. |
We currently have directors’ and officers’ liability insurance, providing total coverage of $10 million. We also have side A directors’ and officers’ liability insurance, providing total coverage of $10 million in excess of the $10 million directors’ and officers’ liability insurance, for the benefit of all of our directors and officers, which expires on July 31, 2021. Upon the listing on Nasdaq, we intend to cancel those policies and to purchase directors’ and officers’ liability insurance to cover the Nasdaq listing exposure (among other exposures) with a limit of not less than $5 million. Such insurance may include side A directors’ and officers’ liability insurance.
Indemnification
The Companies Law and the Israeli Securities Law, 5728-1968, or the Securities Law, provide that a company may indemnify an office holder against the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking made in advance of an event or following an event, provided its articles of association include a provision authorizing such indemnification:
102
| ● | a financial liability imposed on him or her in favor of another person by any judgment concerning an act performed in his or her capacity as an office holder, including a settlement or arbitrator’s award approved by a court; |
| ● | reasonable litigation expenses, including attorneys’ fees, expended by the office holder (a) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (1) no indictment (as defined in the Companies Law) was filed against such office holder as a result of such investigation or proceeding; and (2) no financial liability as a substitute for the criminal proceeding (as defined in the Companies Law) was imposed upon him or her as a result of such investigation or proceeding, or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; or (b) in connection with a monetary sanction; |
| ● | reasonable litigation expenses, including attorneys’ fees, expended by the office holder or imposed on him or her by a court: (1) in proceedings that the company institutes, or that another person institutes on the company’s behalf, against him or her; (2) in a criminal proceedings of which he or she was acquitted; or (3) as a result of a conviction for a crime that does not require proof of criminal intent; and |
| ● | expenses incurred by an office holder in connection with an Administrative Procedure under the Securities Law, including reasonable litigation expenses and reasonable attorneys’ fees. An “Administrative Procedure” is defined as a procedure pursuant to chapters H3 (Monetary Sanction by the Israeli Securities Authority), H4 (Administrative Enforcement Procedures of the Administrative Enforcement Committee) or I1 (Arrangement to prevent Procedures or Interruption of procedures subject to conditions) to the Securities Law. |
The Companies Law also permits a company to undertake in advance to indemnify an office holder, provided that if such indemnification relates to financial liability imposed on him or her, as described above, then the undertaking should be limited and shall detail the following foreseen events and amount or criterion:
| ● | to events that in the opinion of the board of directors can be foreseen based on the company’s activities at the time that the undertaking to indemnify is made; and |
| ● | in amount or criterion determined by the board of directors, at the time of the giving of such undertaking to indemnify, to be reasonable under the circumstances. |
We have entered into indemnification agreements with all of our directors and with all members of our senior management. Each such indemnification agreement provides the office holder with indemnification permitted under applicable law and up to a certain amount, and to the extent that these liabilities are not covered by directors and officer's insurance.
Exculpation
Under the Companies Law, an Israeli company may not exculpate an office holder from liability for a breach of his or her duty of loyalty, but may exculpate in advance an office holder from his or her liability to the company, in whole or in part, for damages caused to the company as a result of a breach of his or her duty of care (other than in relation to distributions), but only if a provision authorizing such exculpation is included in its articles of association. Our amended and restated articles of association provide that we may exculpate, in whole or in part, any office holder from liability to us for damages caused to the company as a result of a breach of his or her duty of care, but prohibit an exculpation from liability arising from a company’s transaction in which our controlling shareholder or officer has a personal interest. Subject to the aforesaid limitations, under the indemnification agreements, we exculpate and release our office holders from any and all liability to us related to any breach by them of their duty of care to us to the fullest extent permitted by law.
103
Limitations
The Companies Law provides that we may not exculpate or indemnify an office holder nor enter into an insurance contract that would provide coverage for any liability incurred as a result of any of the following: (1) a breach by the office holder of his or her duty of loyalty unless (in the case of indemnity or insurance only, but not exculpation) the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice us; (2) a breach by the office holder of his or her duty of care if the breach was carried out intentionally or recklessly (as opposed to merely negligently); (3) any act or omission committed with the intent to derive an illegal personal benefit; or (4) any fine, monetary sanction, penalty or forfeit levied against the office holder.
Under the Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to certain office holders or under certain circumstances, also by the shareholders.
Our amended and restated articles of association permit us to exculpate (subject to the aforesaid limitation), indemnify and insure our office holders to the fullest extent permitted or to be permitted by the Companies Law.
The foregoing descriptions summarize the material aspects and practices of our board of directors. For additional details, we also refer you to the full text of the Companies Law, as well as of our amended and restated articles of association, which are exhibits to this registration statement of which this prospectus forms a part, and are incorporated herein by reference.
There are no service contracts between us or any of our subsidiaries, on the one hand, and our directors in their capacity as directors, on the other hand, providing for benefits upon termination of service.
Approval of Related Party Transactions under Israeli Law
General
Under the Companies Law, we may approve an action by an office holder from which the office holder would otherwise have to refrain, as described above, if:
| ● | the office holder acts in good faith and the act or its approval does not cause harm to the company; and |
| ● | the office holder disclosed the nature of his or her interest in the transaction (including any significant fact or document) to the company at a reasonable time before the company’s approval of such matter. |
Disclosure of Personal Interests of an Office Holder
The Companies Law requires that an office holder disclose to the company, promptly, and, in any event, not later than the board meeting at which the transaction is first discussed, any direct or indirect personal interest that he or she may have and all related material information known to him or her relating to any existing or proposed transaction by the company, including without limitations, any material document or fact regarding such transaction. If the transaction is an extraordinary transaction, the office holder must also disclose any personal interest held by:
| ● | the office holder’s relatives; or |
| ● | any corporation in which the office holder or his or her relatives holds 5% or more of the shares or voting rights, serves as a director or general manager or has the right to appoint at least one director or the general manager. |
104
An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction. Under the Companies Law, an extraordinary transaction is a transaction:
| ● | not in the ordinary course of business; or |
| ● | not on market terms; or |
| ● | that is likely to have a material effect on the company’s profitability, assets or liabilities. |
The Companies Law does not specify to whom within us nor the manner in which required disclosures are to be made. We require our office holders to make such disclosures to our board of directors.
Under the Companies Law, once an office holder complies with the above disclosure requirement, the board of directors may approve a transaction between the company and an office holder, or a third party in which an office holder has a personal interest, unless the articles of association provide otherwise and provided that the transaction is in the company’s interest. If the transaction is an extraordinary transaction in which an office holder has a personal interest, first the audit committee and then the board of directors, in that order, must approve the transaction. Under specific circumstances, shareholder approval may also be required. Generally, a person who has a personal interest in a matter which is considered at a meeting of the board of directors or the audit committee may not be present at such a meeting unless the chairman of the audit committee or board of directors (as applicable) determines that he or she should be present in order to present the transaction that is subject to approval. A director who has a personal interest in a transaction, which is considered at a meeting of the board of directors or the audit committee, may not be present at this meeting or vote on this matter, unless a majority of members of the board of directors or the audit committee, as the case may be, has a personal interest. If a majority of the board of directors has a personal interest, then shareholder approval is generally also required.
Disclosure of Personal Interests of a Controlling Shareholder
Under the Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, and transactions concerning the terms of engagement and compensation of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee, require the approval of the audit committee or the compensation committee, as the case may be, the board of directors and a majority of the shares voted by the shareholders of the company participating and voting on the matter in a shareholders’ meeting. In addition, the shareholder approval must fulfill one of the following requirements, or a Special Majority:
| ● | at least a majority of the shares held by shareholders who are not controlling shareholders and have no personal interest in the transaction and are voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or |
| ● | the shares voted by shareholders who vote against the transaction represent no more than 2% of the voting rights in the company. |
In addition, any extraordinary transaction with a controlling shareholder or in which a controlling shareholder has a personal interest with a term of more than three years requires the abovementioned approval every three years; however, such transactions not involving the receipt of services or compensation can be approved for a longer term, provided that the audit committee determines that such longer term is reasonable under the circumstances.
The Companies Law requires that every shareholder that participates, in person, by proxy or by voting instrument, in a vote regarding a transaction with a controlling shareholder, must indicate in advance or in the ballot whether or not that shareholder has a personal interest in the vote in question. Failure to indicate such personal interest will result in the invalidation of that shareholder’s vote.
105
The term “controlling shareholder” is defined in the Companies Law as a shareholder with the ability to direct the activities of the company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in a company or has the right to appoint 50% or more of the directors of the company or its general manager. In the context of a transaction involving a shareholder of the company, a controlling shareholder also includes a shareholder who holds 25% or more of the voting rights in the company if no other shareholder holds more than 50% of the voting rights in the company. For this purpose, the holdings of all shareholders who have a personal interest in the same transaction will be aggregated.
Approval of the Compensation of Directors and Executive Officers
The compensation of, or an undertaking to indemnify, insure or exculpate, an office holder who is not a director requires the approval of the company’s compensation committee, followed by the approval of the company’s board of directors, and, if such compensation arrangement or an undertaking to indemnify, insure or exculpate is inconsistent with the company’s stated compensation policy, or if the said office holder is the chief executive officer of the company (subject to a number of specific exceptions), then such arrangement is subject to the approval of our shareholders, subject to a Special Majority requirement.
Directors. Under the Companies Law, the compensation of our directors requires the approval of our compensation committee, the subsequent approval of the board of directors and, unless exempted under the regulations promulgated under the Companies Law, the approval of the general meeting of our shareholders. If the compensation of our directors is inconsistent with our stated compensation policy, then, provided that those provisions that must be included in the compensation policy according to the Companies Law have been considered by the compensation committee and board of directors, shareholder approval by a Special Majority will be required.
Executive officers other than the chief executive officer. The Companies Law requires the approval of the compensation of a public company’s executive officers (other than the chief executive officer) in the following order: (i) the compensation committee, (ii) the company’s board of directors, and (iii) only if such compensation arrangement is inconsistent with the company’s stated compensation policy, the company’s shareholders by a Special Majority. However, if the shareholders of the company do not approve a compensation arrangement with an executive officer that is inconsistent with the company’s stated compensation policy, the compensation committee and board of directors may override the shareholders’ decision if each of the compensation committee and the board of directors provide detailed reasons for their decision, including regarding to the shareholders of the Company objection.
Chief executive officer. Under the Companies Law, the compensation of a public company’s chief executive officer is required to be approved by: (i) the company’s compensation committee; (ii) the company’s board of directors, and (iii) the company’s shareholders by a Special Majority. However, if the shareholders of the company do not approve the compensation arrangement with the chief executive officer, the compensation committee and board of directors may override the shareholders’ decision if each of the compensation committee and the board of directors provides detailed reasons for their decision. In addition, the compensation committee may exempt the engagement terms of a candidate to serve as the chief executive officer from shareholders’ approval, if the compensation committee determines that the compensation arrangement is consistent with the company’s stated compensation policy, that the chief executive officer did not have a prior business relationship with the company or a controlling shareholder of the company, and that subjecting the approval to a shareholder vote would impede the company’s ability to attain the candidate to serve as the company’s chief executive officer (and provide detailed reasons for the latter).
The approval of each of the compensation committee and the board of directors, with regard to the office holders and directors above, must be in accordance with the company’s stated compensation policy; however, under special circumstances, the compensation committee and the board of directors may approve compensation terms of a chief executive officer that are inconsistent with the company’s compensation policy provided that they have considered those provisions that must be included in the compensation policy according to the Companies Law and that shareholder approval was obtained by a Special Majority requirement.
Duties of Shareholders
Under the Companies Law, a shareholder has a duty to refrain from abusing his power in the company and to act in good faith and in an acceptable manner in exercising his rights and performing his obligations toward the company and other shareholders, including, among other things, in voting at general meetings of shareholders (and at shareholder class meetings) on the following matters:
106
| ● | amendment of the articles of association; |
| ● | increase in the company’s authorized share capital; |
| ● | merger; and |
| ● | the approval of related party transactions and acts of office holders that require shareholder approval. |
A shareholder also has a general duty to refrain from oppressing other shareholders. The remedies generally available upon a breach of contract will also apply to a breach of the above-mentioned duties, and in the event of oppression of other shareholders, additional remedies are available to the injured shareholder.
In addition, any controlling shareholder, any shareholder that knows that its vote can determine the outcome of a shareholder vote and any shareholder that, under a company’s articles of association, has the power to appoint or prevent the appointment of an office holder, or has another power with respect to a company, is under a duty to act with fairness towards the company. The Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account.
Equity Incentive Plan
2017 Stock Option Plan
We maintain one equity incentive plan – our Employee Stock Option Plan (2017), or the 2017 Plan. July 6, 2021, the number of options allotted are 11,785,912. In addition. The number of options that have vested and have not yet been exercised or expired are 6,608,049.
Our 2017 Plan was adopted by our board of directors in March 2017, and expires on March 2027. Our employees, directors, officer, consultants, advisors, suppliers and any other person or entity whose services are considered valuable to us are eligible to participate in this plan.
Our 2017 Plan is administered by our board of directors, regarding the granting of options and the terms of option grants, including exercise price, method of payment, vesting schedule, acceleration of vesting and the other matters necessary in the administration of these plan. Eligible Israeli employees, officers and directors, would qualify for provisions of Section 102(b)(2) of the Israeli Income Tax Ordinance (New Version), 5721–1961, or the Tax Ordinance. Pursuant to such Section 102(b)(2), qualifying options and shares issued upon exercise of such options are held in trust and registered in the name of a trustee selected by the board of directors. The trustee may not release these options or shares to the holders thereof for two years from the date of the registration of the options in the name of the trustee. Under Section 102, any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or ordinary shares by the trustee to the employee or upon the sale of the options or ordinary shares, and gains may qualify to be taxed as capital gains at a rate equal to 25%, subject to compliance with specified conditions. Our Israeli non-employee service providers and controlling shareholders may only be granted options under Section 3(9) of the Tax Ordinance, which does not provide for similar tax benefits. The 2017 Plan also permits the grant to Israeli grantees of options that do not qualify under Section 102(b)(2).
Upon termination of employment without Cause, as defined in the 2017 Plan, all unvested options will expire, and all vested options will generally be exercisable for three (3) months following termination, or such other period as determined by the plan administrator, subject to the terms of the 2017 Plan and the governing option agreement.
Upon termination of employment due to death, retirement or disability, all the vested options at the time of termination will be exercisable for twenty four (24) months following termination, or such other period as determined by the plan administrator, subject to the terms of the 2017 Plan and the governing option agreement.
107
BENEFICIAL OWNERSHIP OF PRINCIPAL SHAREHOLDERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our Ordinary Shares as of July 6, 2021 by:
| ● | each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our outstanding ordinary shares; |
| ● | each of our directors and executive officers; and |
| ● | all of our directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC, and includes voting or investment power with respect to Ordinary Shares. Ordinary Shares issuable under share options or warrants that are exercisable within 60 days after July 6, 2021, are deemed outstanding for the purpose of computing the percentage ownership of the person holding the options or warrants but are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
As of July 6, 2021, there was one holder of record of our Ordinary Shares. The number of record holders is not representative of the number of beneficial holders of our Ordinary Shares, as the shares of most our shareholders who hold Ordinary Shares that are traded on the TASE are recorded in the name of our Israeli share registrar, Bank REGISTRATION CO. OF UNITED MIZRAHI BANK LTD. As of July 6, 2021, we had no record holders of our Ordinary Shares in the United States.
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to us which would result in a change in control of our company at a subsequent date. Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. Unless otherwise noted below, each beneficial owner’s address is: c/o IceCure Medical Ltd., 7 Ha’Eshel St., PO Box 3163, Caesarea, 3079504 Israel.
| No. of Shares Beneficially Owned | Percentage Owned | |||||||
| Holders of more than 5% of our voting securities: | ||||||||
| Epoch Partner Investments Limited (1) | 141,067,059 | 55.5 | % | |||||
| Clover Wolf Capital Limited Partnership (2) | 18,989,681 | 7.47 | % | |||||
| Clover Alpha L.P. (3) | 2,450,282 | 0.96 | % | |||||
| Alpha Capital Anstalt (4) | 24,502,815 | 9.64 | % | |||||
| Directors and senior management who are not 5% holders: | ||||||||
| Ron Mayron * | 779,567 | 0.31 | % | |||||
| Eyal Shamir * | 2,481,425 | 0.97 | % | |||||
| Ronen Tsimerman | 1,341,415 | 0.52 | % | |||||
| Shay Levav | - | - | ||||||
| Tlalit Bussi Tel-Tzure | 397,600 | 0.16 | % | |||||
| Naum Muchnik | 693,895 | 0.27 | % | |||||
| Doron Birger * | - | - | ||||||
| Sharon Levita * | - | - | ||||||
| Oded Tamir * | - | - | ||||||
| Yang Huang * | - | - | ||||||
| All directors and senior management as a group (10 persons) | 5,693,902 | 2.19 | % | |||||
| (1) | Includes 141,067,059 Ordinary Shares. Mr. Li Haixiang has the voting and dispositive power over the shares held by Epoch Partner Investments Limited. The mailing address of Mr. Li Haixiang is 70/F Two International Finance Centere, Suite 7013, Central, Hong Kong. |
| (2) | Includes 18,989,681 Ordinary Shares. Ms. Adi Wolf has the voting and dispositive power over the shares held by Clover Wolf Capital Limited Partnership. The mailing address of Ms. Adi Wolf is 24 Bodenheimer, Tel Aviv 6200838, Israel. |
| (3) | Includes 2,450,282 Ordinary Shares. Ms. Adi Wolf has the voting and dispositive power over the shares held by Clover Alpha L.P. The mailing address of Ms. Adi Wolf is 24 Bodenheimer, Tel Aviv 6200838, Israel. |
| (4) | Includes 24,502,815 Ordinary Shares. Mr. Konard Ackermann, Dr. Alexander Lins and Dr. Nicola Feuerstein have the voting and dispositive power over the shares held by Alpha Capital Anstalt. The mailing address of Mr. Konard Ackermann, Dr. Alexander Lins and Dr. Nicola Feuerstein is Lettstrasse 32, FL-9490 Vaduz, Liechtenstein. |
| * | Indicates director of the Company. |
|
** |
Less than 1%. |
108
Changes in Percentage Ownership by Major Shareholders
On February 7, 2015, we entered into the Epoch SPA pursuant to which we received $5,485 thousand against issuance of 15,142,857 Ordinary Shares and 2,271,429 warrants. The warrants were not exercised and expired. Prior to the Epoch SPA, Epoch Partner Investments Limited, did not hold shares of our issued and outstanding share capital, and following the Epoch SPA, Epoch Partner Investments Limited held approximately 57.13% of our issued and outstanding share capital.
Since the Epoch SPA we performed multiple public and rights offerings. On June 20, 2017 and on August 25, 2017 the company received two favorable loans of $500 thousand each from our controlling shareholder, Epoch Partner Investments Limited, or the Epoch Loans, baring interest equal the interest of U.S. government bond for one year. Following a rights offering that was completed on February 14, 2018, and in which Epoch Partner Investments Limited exercised rights at an aggregate amount of $3,898 thousand, we repaid the Epoch Loans plus $8 thousand interest. Following this rights offering, Epoch Partner Investments Limited held 68.03% of our issued and outstanding share capital.
On May 15, 2018 we held a public offering in which Epoch Partner Investments Limited didn’t participate. As a result, it’s holding decreased to 62.58% of our issued and outstanding share capital.
On February 20, 2019 we held a public offering in which Epoch Partner Investments participated and invested $464 thousand against issuance of 2,000,000 shares. Following the offering, Epoch Partner Investments Limited held approximately 61.94% of our issued and outstanding share capital.
On October 7, 2019, we finalized a rights offering in which Epoch Partner Investments Limited exercised its rights for $2,008 thousand against issuance of 11,912,960 shares. Following that, Epoch Partner Investments Limited held approximately 64.43% of our issued and outstanding share capital.
On August 5, 2020 we held a public offering in which Epoch Partner Investments Limited participated and invested $2,592 thousand against issuance of 17,689,997 shares. Following the offering, Epoch Partner Investments Limited held approximately 58.83% of our issued and outstanding share capital.
On January 26, 2021, we entered into the January 2021 SPA. Prior to the January 2021 SPA, our controlling shareholder, Epoch Partner Investments Limited, held approximately 58.62% of our issued and outstanding share capital, and following the January 2021 SPA, Epoch Partner Investments Limited will hold approximately 55.5% of our issued and outstanding share capital.
Record Holders
As of March 15, 2021, there was one shareholder of record of our Ordinary Shares, which was located in Israel. The number of record holders is not representative of the number of beneficial holders of our Ordinary Shares, as the shares of all shareholders for a publicly traded company such as ours which is listed on the Tel Aviv Stock Exchange are recorded in the name of our Israeli share registrar, REGISTRATION CO. OF UNITED MIZRAHI BANK LTD.
The Company is not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to the Company which would result in a change in control of the Company at a subsequent date.
109
Employment Agreements
We have entered into written employment agreements with each of our executive officers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we have entered into agreements with each executive officer and director pursuant to which we have agreed to indemnify each of them up to a certain amount and to the extent that these liabilities are not covered by directors and officers insurance. Members of our senior management are eligible for bonuses each year. The bonuses are payable upon meeting objectives and targets that are set by our Chief Executive Officer and approved annually by our board of directors that also set the bonus targets for our Chief Executive Officer.
Options
Since our inception of the 2017 Plan, we have granted options to purchase our Ordinary Shares to our officers and certain of our directors. Such option agreements may contain acceleration provisions upon certain merger, acquisition, or change of control transactions, as defined in the 2017 Plan or in the stated compensation policy, as the case may be. We describe our option plans under “Management—Equity Incentive Plan.” If the relationship between us and an executive officer or a director is terminated, except for Cause (as defined in the various option plan agreements and the 2017 Plan), options that are vested will generally remain exercisable for three months after such termination.
Private Placement of Ordinary Shares
On January 26, 2021 we entered into the January 2021 SPA, with certain investors, including our controlling shareholder, Epoch Partner Investments Limited, pursuant to which we issued an aggregate of 91,885,555 Ordinary Shares for an aggregate purchase price of $15 million, of which 45,942,777 Ordinary Shares were issued to Epoch Partner Investments Limited.
110
The 91,885,555 Ordinary Shares being offered by the selling shareholders are those previously issued to the selling shareholders. For additional information regarding the issuances of the Ordinary Shares see “Prospectus Summary—Recent Private Placement”. We are registering the Ordinary Shares in order to permit the selling shareholders to offer the Ordinary Shares for resale from time to time.
One of the Selling Shareholders, Epoch Partner Investments Limited, holds approximately 55.5% of the Company’s voting rights and is a controlling shareholder of the Company (see “Beneficial Ownership of Principal Shareholders and Management”). Other than the relationships described herein, to our knowledge, the selling shareholders have not had any material relationship with us within the past three years.
Any selling shareholders that are affiliates of broker-dealers and any participating broker-dealers would be deemed to be “underwriters” within the meaning of the Securities Act, and any commissions or discounts given to any such selling shareholders or broker-dealer may be regarded as underwriting commissions or discounts under the Securities Act. To our knowledge, none of the selling shareholders listed below are broker-dealers or affiliates of broker-dealers.
The table below lists the selling shareholders and other information regarding the beneficial ownership of the Ordinary Shares by each of the selling shareholders. The second column lists the number of Ordinary Shares beneficially owned by each selling shareholder, based on its ownership of the Ordinary Shares, as of July 6, 2021.
The third column lists the Ordinary Shares being offered by this prospectus by the selling shareholders.
In accordance with the terms of a registration rights agreement with the holders of the Ordinary Shares, this prospectus generally covers the resale of at least a number of Ordinary Shares issued. Because the number of Ordinary Shares may be adjusted, the number of Ordinary Shares that will actually be issued may be more or less than the number of Ordinary Shares being offered by this prospectus. The fourth column assumes the sale of all of the Ordinary Shares offered by the selling shareholders pursuant to this prospectus.
| Name of Selling Shareholders | Ordinary
Shares Beneficially Owned Prior to Offering (1) | Percentage of Existing Equity Capital | Maximum Number of Ordinary Shares to be Sold Pursuant to this Prospectus | Ordinary Shares Owned Immediately After Sale of Maximum Number of Shares in this Offering | Percentage
of | |||||||||||||||
| Epoch Partners Investments Limited (2) | 141,067,059 | 55.5 | % | 45,942,777 | 95,124,282 | 37.43 | % | |||||||||||||
| Clover Wolf Capital limited partnership (3) | 18,989,681 | 7.47 | % | 18,989,681 | 0 | - | ||||||||||||||
| Clover Alpha L.P. (4) | 2,450,282 | 0.96 | % | 2,450,282 | 0 | - | ||||||||||||||
| Alpha Capital Anstalt (5) | 24,502,815 | 9.64 | % | 24,502,815 | 0 | - | ||||||||||||||
| Total | 187,009,837 | 73.57 | % | 91,885,555 | 95,124,282 | - | ||||||||||||||
| (1) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. Ordinary Shares subject to options or warrants currently exercisable, or exercisable within 60 days of July 6, 2021, are counted as outstanding for computing the percentage of the selling shareholder holding such options or warrants but are not counted as outstanding for computing the percentage of any other selling shareholder. |
| (2) | Mr. Li Haixiang has the voting and dispositive power over the shares held by Epoch Partner Investments Limited. |
| (3) | Ms. Adi Wolf has the voting and dispositive power over the shares held by Clover Wolf Capital Limited Partnership. |
| (4) | Ms. Adi Wolf has the voting and dispositive power over the shares held by Clover Alpha L.P. |
| (5) | Mr. Konard Ackermann, Dr. Alexander Lins and Dr. Nicola Feuerstein have the voting and dispositive power over the shares held by Alpha Capital Anstalt. |
| * | Indicates director of the Company. |
111
We are registering the Ordinary Shares previously issued, to permit the resale of these Ordinary Shares by the holders of these securities from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the selling shareholders of the Ordinary Shares. Unlike an initial public offering, any resale by the selling shareholders of the Ordinary Shares is not being underwritten by any investment bank. We will bear all fees and expenses incident to our obligation to register the Ordinary Shares.
The selling shareholders may sell all or a portion of the Ordinary Shares beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the Ordinary Shares are sold through underwriters or broker-dealers, the selling shareholders will be responsible for underwriting discounts or commissions or agent’s commissions. The Ordinary Shares may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions,
| ● | on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| ● | in the over-the-counter market; |
| ● | in transactions other than on these exchanges or systems or in the over-the-counter market; |
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; |
| ● | privately negotiated transactions; |
| ● | sales pursuant to Rule 144 under the Securities Act; |
| ● | broker-dealers may agree with the selling securityholders to sell a specified number of such shares at a stipulated price per share; |
| ● | a combination of any such methods of sale; and |
| ● | any other method permitted pursuant to applicable law. |
If the selling shareholders effect such transactions by selling Ordinary Shares to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling shareholders or commissions from purchasers of the Ordinary Shares for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved).
The selling shareholders may pledge or grant a security interest in some or all of the Ordinary Shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the Ordinary Shares from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act, amending, if necessary, the list of selling shareholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling shareholders also may transfer and donate the Ordinary Shares in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus. The selling shareholders and any broker-dealer participating in the distribution of the shares may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of Ordinary Shares being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling shareholders and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
112
Under the securities laws of some states, the Ordinary Shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the Ordinary Shares may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling shareholder will sell any or all of the Ordinary Shares registered pursuant to the registration statement, of which this prospectus forms a part.
The selling shareholders and any other person participating in such distribution will be subject to applicable provisions of the Securities Exchange, and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares by the selling shareholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the Ordinary Shares to engage in market-making activities with respect to the shares. All of the foregoing may affect the marketability of the Ordinary Shares and the ability of any person or entity to engage in market-making activities with respect to the Ordinary Shares.
We will pay all expenses of the registration of the Ordinary Shares pursuant to the registration rights agreement, estimated to be $ in total, including, without limitation, SEC filing fees and expenses of compliance with state securities or “blue sky” laws; provided, however, that a selling shareholder will pay all underwriting discounts and selling commissions, if any. We will indemnify the selling shareholders against liabilities, including some liabilities under the Securities Act, in accordance with the registration rights agreements, or the selling shareholders will be entitled to contribution. We may be indemnified by the selling shareholders against civil liabilities, including liabilities under the Securities Act, that may arise from any written information furnished to us by the selling shareholders specifically for use in this prospectus, in accordance with the related registration rights agreement, or we may be entitled to contribution.
Once sold under the registration statement, of which this prospectus forms a part, the Ordinary Shares will be freely tradable in the hands of persons other than our affiliates.
113
DESCRIPTION OF SHARE CAPITAL AND GOVERNING DOCUMENTS
General
As of July 6, 2021, our authorized share capital consisted of 20,000,000,000 Ordinary Shares, with no par value, of which 254,183,480 shares were issued and outstanding as of such date. All of our outstanding Ordinary Shares have been validly issued, fully paid and non-assessable. Our Ordinary Shares are not redeemable and are not subject to any preemptive right.
Our registration number with the Israeli Registrar of Companies is 513787804.
Ordinary Shares
In the last three years, we have issued an aggregate of 152,629,769 Ordinary Shares in several public offerings, rights offerings and exercise of employees’ stock options for aggregate net proceeds of $24,232 thousand (in each case based on the exchange rate of the NIS and U.S. dollar applicable on the day of the closing of the respective transaction) thousand.
Options
In the last three years, we have granted options to purchase an aggregate of 8,860,366 Ordinary Shares to directors, officers and employees with exercise prices ranging from NIS 0.605 to NIS 2.24 (approximately $0.18 to $0.69) per share A total of 752,656 options were exercised in the last three years.
Our Articles of Association
Purposes and Objects of the Company
Our purpose is set forth in Article 4 of our articles of association and includes every lawful purpose.
The Powers of the Directors
Our board of directors shall direct our policy and shall supervise the performance of our Chief Executive Officer and his actions. Our board of directors may exercise all powers that are not required under the Companies Law or under our amended and restated articles of association to be exercised or taken by our shareholders.
Rights Attached to Shares
Our Ordinary Shares shall confer upon the holders thereof:
| ● | equal right to attend and to vote at all of our general meetings, whether regular or special, with each Ordinary Share entitling the holder thereof, which attend the meeting and participate at the voting, either in person or by a proxy or by a written ballot, to one vote; |
| ● | equal right to participate in distribution of dividends, if any, whether payable in cash or in bonus shares, in distribution of assets or in any other distribution, on a per share pro rata basis; and |
| ● | equal right to participate, upon our dissolution, in the distribution of our assets legally available for distribution, on a per share pro rata basis. |
114
Election of Directors
Pursuant to our amended and restated articles of association, our directors are elected at an annual general meeting and/or a special meeting of our shareholders and serve on the board of directors until the next annual general meeting (except for external directors) or until they resign or until they cease to act as board members pursuant to the provisions of our amended and restated articles of association or any applicable law, upon the earlier. Pursuant to the Companies Law, other than the external directors, for whom special election requirements apply under the Companies Law, the vote required to appoint a director is a simple majority vote of holders of our voting shares, participating and voting at the relevant meeting. In addition, our amended and restated articles of association allow our board of directors to appoint directors to fill vacancies and/or as an addition to the board of directors (subject to the maximum number of directors) to serve until the next annual general meeting. External directors are elected for an initial term of three years, may be elected for additional terms of three years each under certain circumstances, and may be removed from office pursuant to the terms of the Companies Law. (see “Management—Board Practices—External Directors”).
Annual and Special Meetings
Under the Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year, at such time and place which shall be determined by our Board of Directors, that must be no later than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders are referred to as special general meetings. Our board of directors may call special meetings whenever it sees fit and upon the request of: (a) any two of our directors or such number of directors equal to one quarter of the directors then at office; and/or (b) one or more shareholders holding, in the aggregate, (i) 5% or more of our outstanding issued shares and 1% of our outstanding voting power or (ii) 5% or more of our outstanding voting power.
Subject to the provisions of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and forty days prior to the date of the meeting, as the case may be. Resolutions regarding the following matters must be passed at a general meeting of our shareholders:
| ● | amendments to our amended and restated articles of association; |
| ● | the exercise of our board of directors’ powers by a general meeting if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management; |
| ● | appointment or termination of our auditors; |
| ● | appointment of directors, including external directors; |
| ● | approval of acts and transactions requiring general meeting approval pursuant to the provisions of the Companies Law (mainly certain related party transactions) and any other applicable law; |
| ● | increases or reductions of our authorized share capital; |
| ● | a merger (as such term is defined in the Companies Law); and |
| ● | a dissolution of the Company by the court or by its shareholders (as such term is defined in the Companies Law). |
Notices
The Companies Law and our amended and restated articles of association require that a notice of any annual or special shareholders meeting be provided at least 14 or 21 days prior to the meeting, as the case may be, and if the agenda of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, approval of the company’s general manager to serve as the chairman of the board of directors or an approval of a merger, notice must be provided at least 35 days prior to the meeting.
115
Quorum
As permitted under the Companies Law, the quorum required for our general meetings consists of at least two shareholders present in person, by proxy, written ballot or voting by means of electronic voting system, who hold or represent between them at least 25% of the total outstanding voting rights. If half an hour has elapsed from the date set for the meeting and the quorum has not been found valid, the meeting will be postponed to the business day after the day of the meeting, to the same time and to the same place or to another day, time and place as determined by the board of directors. The company will announce through the immediate report of the postponement of the meeting and the date of the postponed meeting. If no lawful quorum is present at the adjourned meeting as aforesaid, at least one shareholder shall be present in person or by proxy, a lawful quorum, unless the meeting was convened at the request of shareholders. If a special general meeting was summoned following the request of a shareholder, and within half an hour a legal quorum shall not have been formed, the meeting shall be canceled.
Adoption of Resolutions
Our amended and restated articles of association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required under the Companies Law or our amended and restated articles of association. A shareholder may vote in a general meeting in person, by proxy, by a written ballot.
Changing Rights Attached to Shares
Unless otherwise provided by the terms of the shares and subject to any applicable law, any modification of rights attached to any class of shares must be adopted by the holders of a majority of the shares of that class present a general meeting of the affected class or by a written consent of all the shareholders of the affected class.
The enlargement of an existing class of shares or the issuance of additional shares thereof, shall not be deemed to modify the rights attached to the previously issued shares of such class or of any other class, unless otherwise provided by the terms of the shares.
Limitations on the Right to Own Securities in Our Company
There are no limitations on the right to own our securities.
Provisions Restricting Change in Control of Our Company
There are no specific provisions of our amended and restated articles of association that would have an effect of delaying, deferring or preventing a change in control of the Company or that would operate only with respect to a merger, acquisition or corporate restructuring involving us (or any of our subsidiaries). However, as described below, certain provisions of the Companies Law may have such effect.
The Companies Law includes provisions that allow a merger transaction and requires that each company that is a party to the merger have the transaction approved by its board of directors and, unless certain requirements described under the Companies Law are met, a vote of the majority of shareholders, and, in the case of the target company, also a majority vote of each class of its shares. For purposes of the shareholder vote of each party, unless a court rules otherwise, the merger will not be deemed approved if shares representing a majority of the voting power present at the shareholders meeting and which are not held by the other party to the merger (or by any person or group of persons acting in concert who holds 25% or more of the voting power or the right to appoint 25% or more of the directors of the other party) vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger will be subject to the same Special Majority approval that governs all extraordinary transactions with controlling shareholders instead. Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that as a result of the merger the surviving company will be unable to satisfy the obligations of any of the parties to the merger, and may further give instructions to secure the rights of creditors. If the transaction would have been approved by the shareholders of a merging company but did not receive the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the petition of holders of at least 25% of the voting rights of a company. For such petition to be granted, the court must find that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders. In addition, a merger may not be completed unless at least (1) 50 days have passed from the time that the requisite proposals for approval of the merger were filed with the Israeli Registrar of Companies by each merging company and (2) 30 days have passed since the merger was approved by the shareholders of each merging company.
116
The Companies Law also provides that, subject to certain exceptions, an acquisition of shares in an Israeli public company must be made by means of a “special” tender offer if as a result of the acquisition (1) the purchaser would become a holder of 25% or more of the voting rights in the company, unless there is already another holder of at least 25% or more of the voting rights in the company or (2) the purchaser would become a holder of 45% or more of the voting rights in the company, unless there is already a holder of more than 45% of the voting rights in the company. These requirements do not apply if, in general, the acquisition (1) was made in a private placement that received shareholders’ approval, subject to certain conditions, (2) was from a holder of 25% or more of the voting rights in the company which resulted in the acquirer becoming a holder of 25% or more of the voting rights in the company, or (3) was from a holder of more than 45% of the voting rights in the company which resulted in the acquirer becoming a holder of more than 45% of the voting rights in the company. A “special” tender offer must be extended to all shareholders. In general, a “special” tender offer may be consummated only if (1) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror and (2) the offer is accepted by a majority of the offerees who notified the company of their position in connection with such offer (excluding the offeror, controlling shareholders, holders of 25% or more of the voting rights in the company or anyone on their behalf, or any person having a personal interest in the acceptance of the tender offer). If a special tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
If, as a result of an acquisition of shares, the acquirer will hold more than 90% of an Israeli company’s outstanding shares or of certain class of shares, the acquisition must be made by means of a tender offer for all of the outstanding shares, or for all of the outstanding shares of such class, as applicable. In general, if less than 5% of the outstanding shares, or of applicable class, are not tendered in the tender offer and more than half of the offerees who have no personal interest in the offer tendered their shares, all the shares that the acquirer offered to purchase will be transferred to it by operation of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of shares. Any shareholders that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may request, by petition to an Israeli court, (i) appraisal rights in connection with a full tender offer, and (ii) that the fair value should be paid as determined by the court, for a period of six months following the acceptance thereof. However, the acquirer is entitled to stipulate, under certain conditions, that tendering shareholders will forfeit such appraisal rights.
Lastly, Israeli tax law treats some acquisitions, such as stock-for-stock exchanges between an Israeli company and a foreign company, less favorably than U.S. tax laws. For example, Israeli tax law may, under certain circumstances, subject a shareholder who exchanges his Ordinary Shares for shares in another corporation to taxation prior to the sale of the shares received in such stock-for-stock swap.
Changes in Our Capital
The general meeting may, by a simple majority vote of the shareholders attending the general meeting:
| ● | increase our registered share capital by the creation of new shares from the existing class or a new class, as determined by the general meeting; |
| ● | cancel any registered share capital which have not been taken or agreed to be taken by any person; |
| ● | consolidate and divide all or any of our share capital into shares of larger nominal value than our existing shares; |
| ● | subdivide our existing shares or any of them, our share capital or any of it, into shares of smaller nominal value than is fixed; and |
| ● | reduce our share capital and any fund reserved for capital redemption in any manner, and with and subject to any incident authorized, and consent required, by the Companies Law. |
117
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our Ordinary Shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign, including Israeli, or other taxing jurisdiction.
ISRAELI TAX CONSIDERATIONS AND GOVERNMENT PROGRAMS
The following is a description of the material Israeli income tax consequences of the ownership of our Ordinary Shares. The following also contains a description of material relevant provisions of the current Israeli income tax structure applicable to companies in Israel, with reference to its effect on us. To the extent that the discussion is based on new tax legislation which has not been subject to judicial or administrative interpretation, there can be no assurance that the tax authorities will accept the views expressed in the discussion in question. The discussion is not intended, and should not be taken, as legal or professional tax advice and is not exhaustive of all possible tax considerations.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our Ordinary Shares. Shareholders should consult their own tax advisors concerning the tax consequences of their particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to corporate tax. As of January 2018 -, the corporate tax rate is 23%. However, the effective tax rate payable by a company that derives income from a “Preferred Enterprise” (as discussed below) may be considerably less. Capital gains derived by an Israeli company are generally subject to the prevailing corporate tax rate.
Capital gains derived by an Israeli resident company are subject to tax at the prevailing corporate tax rate. Under Israeli tax legislation, a corporation will be considered as an “Israeli resident company” if it meets one of the following: (i) it was incorporated in Israel; or (ii) the control and management of its business are exercised in Israel.
Law for the Encouragement of Industry (Taxes), -1969
The Encouragement of Industry (Taxes) Law, 5729-1969, generally referred to as the Industry Encouragement Law, provides several tax benefits for “Industrial Companies.”
The Industry Encouragement Law defines an “Industrial Company” as an Israeli resident-company, of which 90% or more of its income in a given tax year, other than income from defense loans, is derived from an “Industrial Enterprise” located in Israel owned by it. An “Industrial Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
| ● | amortization of the cost of purchased a patent, rights to use a patent, and know-how, which are used for the development or advancement of the company, over an eight-year period, commencing on the year in which such rights were first exercised; |
| ● | under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and |
| ● | expenses related to a public offering are deductible in equal amounts over three years. |
Eligibility for benefits under the Industry Encouragement Law is not contingent upon approval of any governmental authority.
118
Tax Benefits and Grants for Research and Development
General. The IIA, an independent publicly funded agency, was created to provide a variety of practical tools and funding platforms aimed at effectively addressing the dynamic and changing needs of the local and international innovation ecosystem. The IIA acts under the Law for the Encouragement of Research, Development and Technological Innovation in the Industry 1984 and the related IIA rules and regulations, or the Innovation Law. Companies that receive funding from the IIA are subject to certain liabilities of the Innovation Law, mainly pertaining to the know-how that was developed with the support of the IIA within the framework of an R&D funding program, and/or its derivatives (herein: “IIA-supported know-how”), and/or to the products derived from the technology that was developed with the support of the IIA within the framework of an R&D funding program, and/or its derivatives, or IIA-supported products.
Ownership Structure. Any change of ownership must be reported to the IIA prior to the execution of the acquisition. A change in the company’s ownership, in which a foreign entity becomes a stakeholder in the company, requires the IIA approval and the new shareholder signature on an undertaking letter acknowledging the company’s liabilities to the Innovation Law.
Royalty payment. Companies supported by the IIA are required to pay royalties on income yielded from the IIA-supported products, until full refund of the grant, which is linked to the US dollar and carries interest (the annual LIBOR interest for annual dollar deposits, as published on the first day of trading of each year, or in an alternate publication according to the Bank of Israel’s announcement). Until July 2017, the rate of the royalties refund was 3% of related income in the first three years, and 3.5% from the 4th year, onward. As of July 2017, the rate of the royalties refund for companies with total revenues of under $70M at the year preceding the application date, has changed to 3%.
Manufacturing location. Until 2003 manufacturing was considered to be done completely in Israel, and after this date, the manufacturing location (including assembly) is determined based on the manufacturing declaration located in the grant application submitted for supporting R&D, or the Manufacturing Declaration. The transfer of manufacturing activity outside Israel may be subject to the prior approval of the IIA and may result in an increased royalty payment’s rate and an increased total royalty payment, which will be calculated based on the deviation from the company’s Manufacturing Declaration. Cumulative deviation of under 10% requires notification of the IIA, while 10% or more requires pre-approval.
The rate of royalty payment due to overseas manufacturing is increased as follows: If the foreign company will be given the rights to only manufacture the IIA-supported products, an additional 1% will be incurred (e.g., instead of paying 3%, the company will pay 4%). However, if the foreign company will be given the rights to both manufacture and distribute the IIA-supported products, the royalties rate may be higher. The increased royalty rate will apply for revenues associated with manufacturing outside of Israel only. In general, royalties will be paid from the final sale price to the client and not from the inter-company transfer price. The company will have to keep paying royalties until it reaches the new royalty liability ceiling.
The increased repayment is calculated according to the percentage of the manufacturing activities that are carried out outside of Israel out of the total cumulative manufacturing activities both in Israel and abroad, as described in the following table:
| Percentage
of manufacturing activities performed outside of Israel, cumulatively |
The increased payment to the Israel Innovation Authority | |
| Up to 50% | 120% of the received grants + interest | |
| 50% – 90% | 150% of the received grants + interest | |
| 90% or more | 300% of the received grants + interest |
Know-how location. To the extent a company wishes to transfer its IIA-supported know-how outside of Israel, it must be preapproved by the IIA and the company may be required to pay an additional payment to the IIA, or the Fee, as described below. This Fee (which also relates to programs that are absolved of royalty payment) is calculated according to the ratio between the total grants received from the IIA and the total financial R&D expenses invested in the related know-how (including the received grants), multiplied by the transaction price of the IIA-supported know-how, or the Basic Amount.
119
The Basic Amount minus the received grants is depreciated at a rate of 1/7 per annum, as of the fourth year from the end of the last supported file in each program. As a result, when transferring IIA-supported know-how after 10 years or more, the maximum payment to the IIA will be only the total sum of the received grants plus interest, minus paid royalties.

However, the aforementioned formula has a minimum and a maximum limits. The minimum amount of the payment is the total sum of grants received plus interest. The maximum amount shall be no higher than 6 times the total sum of grants received plus interest. In the case that the IIA-supported company retains its R&D center in Israel for at least 3 consecutive years, following the year of transferring the IIA-supported know-how outside of Israel, while maintaining at least 75% of its R&D employment in Israel – the payment will be limited to 3 times the total sum of grants received plus interest.
Transferring IIA-supported know-how outside of Israel according to the Innovation Law (including paying the Fee where necessary) releases the IIA-supported company from all liabilities to the IIA.
Transfer of know-how to another Israeli entity is subject to signature of the recipient Israeli entity on a formal IIA issued undertaking document, to comply with the provisions of the Innovation Law, including the restrictions on the transfer of know-how and the obligation to pay royalties.
According to the above, these liabilities should be taken into account when we consider to outsource manufacturing, engage in change of control transactions or otherwise transfer our know-how outside of Israel, and may require us to obtain the pre-approval of the IIA for certain actions and transactions and pay additional payments to the IIA. In particular, any change of control and any change of ownership of our Ordinary Shares that would make a non-Israeli citizen or resident an “interested party,” as defined in the Innovation Law, requires a prior written notice to the IIA in addition to any payment that may be required of us for transfer of manufacturing or know-how outside of Israel. If we fail to comply with the Innovation Law, we may be subject to criminal charges.
Tax Benefits for Research and Development
Israeli tax law allows, under certain conditions, a tax deduction for expenditures, including capital expenditures, for the year in which they are incurred. Expenditures are deemed related to scientific research and development projects, if:
| ● | The expenditures are approved by the relevant Israeli government ministry, determined by the field of research; |
| ● | The research and development must be for the promotion of the company; and |
| ● | The research and development is carried out by or on behalf of the company seeking such tax deduction. |
The amount of such deductible expenses is reduced by the sum of any funds received through government grants for the finance of such scientific research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is related to an expense invested in an asset depreciable under the general depreciation rules of the Tax Ordinance. Expenditures not so approved are deductible in equal amounts over three years.
From time to time, we may apply the IIA for approval to allow a tax deduction for all research and development expenses during the year incurred. There can be no assurance that such application will be accepted.
120
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. A non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company might be exempt from Israeli tax upon meeting the following terms: (1) the shares sold were purchased after January 1, 2009; (2) the capital gain is not derived from the permanent establishment of the foreign resident in Israel; (3) the purchase of shares was not from a relative; (4) and the shares are not traded on the stock exchange.
Additionally, a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, under Convention Between the Government of the United States of America and the Government of the State of Israel with respect to Taxes on Income, as amended, or the United States-Israel Tax Treaty, the sale, exchange or other disposition of shares by a shareholder who is a United States resident (for purposes of the treaty) holding the shares as a capital asset and is entitled to claim the benefits afforded to such a resident by the U.S.-Israel Tax Treaty, or a Treaty U.S. Resident, is generally exempt from Israeli capital gains tax unless: (i) the capital gain arising from such sale, exchange or disposition is attributed to real estate located in Israel; (ii) the capital gain arising from such sale, exchange or disposition is attributed to royalties; (iii) the capital gain arising from the such sale, exchange or disposition is attributed to a permanent establishment in Israel, under certain terms; (iv) such Treaty U.S. Resident holds, directly or indirectly, shares representing 10% or more of the voting capital during any part of the 12-month period preceding the disposition, subject to certain conditions; or (v) such Treaty U.S. Resident is an individual and was present in Israel for 183 days or more during the relevant taxable year.
In some instances where our shareholders may be liable for Israeli tax on the sale of their Ordinary Shares, the payment of the consideration may be subject to the withholding of Israeli tax at source. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents are generally subject to Israeli income tax on the receipt of dividends paid on our Ordinary Shares at the rate of 25%, which tax will be withheld at source, unless relief is provided in a treaty between Israel and the shareholder’s country of residence. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any time during the preceding twelve months, the applicable tax rate is 30%. A “substantial shareholder” is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right. However, a distribution of dividends to non-Israeli residents is subject to withholding tax at source at a rate of 20% if the dividend is distributed from income attributed to a Preferred Enterprise, unless a reduced tax rate is provided under an applicable tax treaty. For example, under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our Ordinary Shares who is a Treaty U.S. Resident is 25%. However, generally, the maximum rate of withholding tax on dividends, not generated by a Preferred Enterprise, that are paid to a United States corporation holding 10% or more of the outstanding voting capital throughout the tax year in which the dividend is distributed as well as during the previous tax year, is 12.5%, provided that not more than 25% of the gross income for such preceding year consists of certain types of dividends and interest. Notwithstanding the foregoing, dividends distributed from income attributed to a “Preferred Enterprise” are not entitled to such reduction under the tax treaty but are subject to a withholding tax rate of 15% for a shareholder that is a U.S. corporation, provided that the condition related to our gross income for the previous year (as set forth in the previous sentence) is met. If the dividend is attributable partly to income derived from a Preferred Enterprise, and partly to other sources of income, the withholding rate will be a blended rate reflecting the relative portions of the two types of income. We cannot assure you that we will designate the profits that we may distribute in a way that will reduce shareholders’ tax liability.
121
U.S. FEDERAL INCOME TAX CONSIDERATIONS
THE FOLLOWING SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF ORDINARY SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the Ordinary Shares. For this purpose, a “U.S. Holder” is a holder of Ordinary Shares that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our Ordinary Shares. This summary generally considers only U.S. Holders that will own our Ordinary Shares as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenues Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, (including with respect to the Tax Cuts and Jobs Act of 2017), and the U.S.-Israel Income Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the IRS with regard to the U.S. federal income tax treatment of an investment in our Ordinary Shares by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity;” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our Ordinary Shares in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our Ordinary Shares as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, Ordinary Shares representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities) or persons who hold Ordinary Shares through a partnership or other pass-through entity are not addressed.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our Ordinary Shares, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Taxation of Dividends Paid on Ordinary Shares
We do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under the heading “Passive Foreign Investment Companies” below and the discussion of “qualified dividend income” below, a U.S. Holder, other than certain U.S. Holder’s that are U.S. corporations, will be required to include in gross income as ordinary income the amount of any distribution paid on Ordinary Shares (including the amount of any Israeli tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution which exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the Ordinary Shares to the extent thereof, and then capital gain. We do not expect to maintain calculations of our earnings and profits under U.S. federal income tax principles and, therefore, U.S. Holders should expect that the entire amount of any distribution generally will be reported as dividend income.
122
In general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, inter alia, dividends received from a “qualified foreign corporation.” A “qualified foreign corporation” is a corporation that is entitled to the benefits of a comprehensive tax treaty with the United States which includes an exchange of information program. The IRS has stated that the U.S.-Israel Tax Treaty satisfies this requirement and we believe we are eligible for the benefits of that treaty.
In addition, our dividends will be qualified dividend income if our Ordinary Shares are readily tradable on the Nasdaq Capital Market or another established securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend is paid or in the prior year, as a PFIC, as described below under “Passive Foreign Investment Companies.” A U.S. Holder will not be entitled to the preferential rate: (1) if the U.S. Holder has not held our Ordinary Shares for at least 61 days of the 121 day period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder is under an obligation to make related payments on substantially similar property. Any days during which the U.S. Holder has diminished its risk of loss on our Ordinary Shares are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who elect to treat the dividend income as “investment income” pursuant to Code section 163(d)(4) will not be eligible for the preferential rate of taxation.
The amount of a distribution with respect to our Ordinary Shares will be measured by the amount of the fair market value of any property distributed, and for U.S. federal income tax purposes, the amount of any Israeli taxes withheld therefrom. Cash distributions paid by us in NIS will be included in the income of U.S. Holders at a U.S. dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such NIS for U.S. federal income tax purposes equal to such U.S. dollar value. If the U.S. Holder subsequently converts the NIS into U.S. dollars or otherwise disposes of it, any subsequent gain or loss in respect of such NIS arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
Taxation of the Disposition of Ordinary Shares
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or other disposition of our Ordinary Shares, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the Ordinary Shares in U.S. dollars and the amount realized on the disposition in U.S. dollar (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of Ordinary Shares will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition. Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses is subject to various limitations.
Passive Foreign Investment Companies
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
| ● | 75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or |
| ● | At least 50% of our assets, averaged over the year and generally determined based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income. |
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income.
The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our Ordinary Shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC.
123
If we currently are or become a PFIC, each U.S. Holder who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our Ordinary Shares at a gain: (1) have such distribution or gain allocated ratably over the U.S. Holder’s holding period for the Ordinary Shares, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the Ordinary Shares while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. We do not intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year. In addition, we do not intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. Therefore, the QEF election will not be available with respect to our Ordinary Shares.
In addition, the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our Ordinary Shares which are regularly traded on a qualifying exchange, including the Nasdaq Capital Market, can elect to mark the Ordinary Shares to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the Ordinary Shares and the U.S. Holder’s adjusted tax basis in the Ordinary Shares. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our Ordinary Shares during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules.
Tax on Net Investment Income
U.S. Holders who are individuals, estates or trusts will generally be required to pay a 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition of our Ordinary Shares), or in the case of estates and trusts on their net investment income that is not distributed. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Tax Consequences for Non-U.S. Holders of Ordinary Shares
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our Ordinary Shares.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our Ordinary Shares or gain from the disposition of our Ordinary Shares if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; or (2) in the case of a disposition of our Ordinary Shares, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
124
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our Ordinary Shares if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding at a rate of 24% with respect to cash dividends and proceeds from a disposition of Ordinary Shares. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Pursuant to recently enacted legislation, a U.S. Holder with interests in “specified foreign financial assets” (including, among other assets, our Ordinary Shares, unless such Ordinary Shares are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file a Report of Foreign Bank and Financial Accounts, or FBAR, if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
Certain legal matters concerning this offering will be passed upon for us by Sullivan & Worcester LLP, New York, New York. Certain legal matters with respect to the legality of the issuance of the securities offered by this prospectus and other legal matters concerning this offering relating to Israeli law will be passed upon for us by Sullivan & Worcester Tel-Aviv (Har-Even & Co.), Tel Aviv, Israel.
The financial statements as of December 31, 2020 and 2019 and for the years then ended included in this prospectus have been so included in reliance upon the report of Brightman Almagor Zohar & Co., a firm in the Deloitte Global Network, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The following are the estimated expenses of the issuance and distribution of the securities being registered under the registration statement of which this prospectus forms a part, all of which will be paid by us. With the exception of the SEC registration fee, all amounts are estimates and may change:
| SEC registration fee | $ | * | ||
| Printer fees and expenses | $ | * | ||
| Legal fees and expenses | $ | * | ||
| Accounting fees and expenses | $ | * | ||
| Miscellaneous | $ | * | ||
| Total | $ | * |
| * | To be filed by amendment. |
125
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in the registration statement of which this prospectus forms a part, a substantial majority of whom reside outside of the United States, may be difficult to obtain within the United States. Furthermore, because substantially all of our assets and a substantial of our directors and officers are located outside of the United States, any judgment obtained in the United States against us or any of our directors and officers may not be collectible within the United States.
We have been informed by our legal counsel in Israel, by Sullivan & Worcester Tel-Aviv (Har-Even & Co.), that it may be difficult to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law.
Subject to specified time limitations and legal procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain exceptions, is non-appealable, including judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that among other things:
| ● | the judgment is obtained after due process before a court of competent jurisdiction, according to the laws of the state in which the judgment is given and the rules of private international law currently prevailing in Israel; |
| ● | the judgment is final and is not subject to any right of appeal; |
| ● | the prevailing law of the foreign state in which the judgment was rendered allows for the enforcement of judgments of Israeli courts; |
| ● | adequate service of process has been effected and the defendant has had a reasonable opportunity to be heard and to present his or her evidence; |
| ● | the liabilities under the judgment are enforceable according to the laws of the State of Israel and the judgment and the enforcement of the civil liabilities set forth in the judgment is not contrary to the law or public policy in Israel nor likely to impair the security or sovereignty of Israel; |
| ● | the judgment was not obtained by fraud and does not conflict with any other valid judgments in the same matter between the same parties; |
| ● | an action between the same parties in the same matter is not pending in any Israeli court at the time the lawsuit is instituted in the foreign court; and |
| ● | the judgment is enforceable according to the laws of Israel and according to the law of the foreign state in which the relief was granted. |
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
126
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act relating to this registration of the Ordinary Shares to be sold by the selling shareholders, or the Registration Statement. This prospectus does not contain all of the information contained in the Registration Statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the Registration Statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the Registration Statement, you may read the document itself for a complete description of its terms.
The SEC also maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
When the Registration Statement is declared effective by the SEC, we will be subject to the information reporting requirements of the Exchange Act, we are subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those requirements are filing reports with the SEC. Those other reports or other information may be inspected without charge at the locations described above. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information.
We maintain a corporate website at http://www.icecure-medical.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference. We will post on our website any materials required to be so posted on such website under applicable corporate or securities laws and regulations, including, posting any XBRL interactive financial data required to be filed with the SEC and any notices of general meetings of our shareholders.
127
91,885,555 Ordinary Shares

IceCure Medical Ltd.
PROSPECTUS
, 2021
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2020
F-1
ICECURE MEDICAL LTD.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2020
TABLE OF CONTENTS
F-2

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of
IceCure Medical Ltd.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of IceCure Medical Ltd. and its subsidiaries (the “Company”) as of December 31, 2020 and 2019, and the related consolidated statements of comprehensive loss, changes in shareholders’ equity and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Brightman Almagor Zohar & Co.
Certified Public Accountants
A Firm in the Deloitte Global Network
Tel Aviv, Israel
May 24, 2021
We have served as the Company’s auditor since 2006.

F-3
CONSOLIDATED BALANCE SHEETS
(U.S. dollars in thousands, except share data and per share data)
| As of December 31, | As of December 31, | |||||||||
| Note | 2020 | 2019 | ||||||||
| ASSETS | ||||||||||
| CURRENT ASSETS | ||||||||||
| Cash and cash equivalents | 3,502 | 5,789 | ||||||||
| Deposit | 3 | 4,669 | — | |||||||
| Trade accounts receivable | 94 | 17 | ||||||||
| Inventory | 4 | 1,064 | 678 | |||||||
| Prepaid expenses and other receivables | 260 | 381 | ||||||||
| Total current assets | 9,589 | 6,865 | ||||||||
| NON-CURRENT ASSETS | ||||||||||
| Prepaid expenses and other long-term assets | 37 | 39 | ||||||||
| Right of use assets | 5 | 306 | 225 | |||||||
| Property and equipment, net | 6 | 307 | 147 | |||||||
| Total non-current assets | 650 | 411 | ||||||||
| TOTAL ASSETS | 10,239 | 7,276 | ||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||
| CURRENT LIABILITIES | ||||||||||
| Trade accounts payable | 645 | 428 | ||||||||
| Lease liabilities | 5 | 214 | 135 | |||||||
| Other current liabilities | 7 | 2,855 | 2,990 | |||||||
| Total current liabilities | 3,714 | 3,553 | ||||||||
| NON-CURRENT LIABILITIES | ||||||||||
| Long term lease liabilities | 5 | 118 | 105 | |||||||
| Other long-term liabilities | 759 | 327 | ||||||||
| Total non-current liabilities | 877 | 432 | ||||||||
| Commitments and contingencies | 8 | |||||||||
| SHAREHOLDERS’ EQUITY | ||||||||||
| Ordinary shares, No par value; Authorized 20,000,000,000 shares; Issued and outstanding: 161,745,754 shares and 120,235,754 shares as of December 31, 2020 and December 31, 2019, respectively | 9 | 3,318 | ||||||||
| Treasury shares | (41 | ) | (41 | ) | ||||||
| Additional paid-in capital | 54,225 | 44,820 | ||||||||
| Accumulated deficit | (48,536 | ) | (44,806 | ) | ||||||
| Total shareholders’ equity | 5,648 | 3,291 | ||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | 10,239 | 7,276 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(U.S. dollars in thousands, except share data and per share data)
| Year ended December 31, | Year ended December 31, | |||||||||
| Note | 2020 | 2019 | ||||||||
| Revenues | 10 | 3,868 | 1,627 | |||||||
| Cost of revenues | 11 | 1,424 | 1,103 | |||||||
| Gross profit | 2,444 | 524 | ||||||||
| Research and development expenses | 12 | 3,809 | 3,001 | |||||||
| Sales and marketing expenses | 13 | 1,063 | 1,035 | |||||||
| General and administrative expenses | 14 | 1,714 | 1,272 | |||||||
| Operating loss | 4,142 | 4,784 | ||||||||
| Financial income, net | (412 | ) | (233 | ) | ||||||
| Net loss and comprehensive loss | 3,730 | 4,551 | ||||||||
| Basic and diluted net loss per share | 0.027 | 0.042 | ||||||||
| Weighted average number of shares outstanding used in computing basic and diluted loss per share | 137,031,221 | 108,970,277 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(U.S. dollars in thousands, except share data and per share data)
| Ordinary shares | Treasury shares | Additional paid-in | Accumulated | Total shareholders’ | ||||||||||||||||||||||||
| Number | Amount | Number | Amount | capital | deficit | equity | ||||||||||||||||||||||
| Balance as of January 1, 2019 | 101,600,445 | 2,787 | (43,465 | ) | (41 | ) | 41,670 | (40,255 | ) | 4,161 | ||||||||||||||||||
| Issuance of ordinary shares, net | 18,635,309 | 531 | - | - | 2,778 | - | 3,309 | |||||||||||||||||||||
| Share-based compensation related to options granted to employees | - | - | - | - | 372 | - | 372 | |||||||||||||||||||||
| Loss for the year | - | - | - | - | - | (4,551 | ) | (4,551 | ) | |||||||||||||||||||
| Balance as of December 31, 2019 | 120,235,754 | 3,318 | (43,465 | ) | (41 | ) | 44,820 | (44,806 | ) | 3,291 | ||||||||||||||||||
| Issuance of ordinary shares, net | 41,400,000 | 1,203 | - | - | 4,644 | - | 5,847 | |||||||||||||||||||||
| Options exercised | 110,000 | 3 | - | - | 8 | - | 11 | |||||||||||||||||||||
| Share-based compensation related to options granted to employees | - | - | - | - | 229 | - | 229 | |||||||||||||||||||||
| Cancelation of par value | - | (4,524 | ) | - | - | 4,524 | - | - | ||||||||||||||||||||
| Loss for the year | - | - | - | - | - | (3,730 | ) | (3,730 | ) | |||||||||||||||||||
| Balance as of December 31, 2020 | 161,745,754 | - | (43,465 | ) | (41 | ) | 54,225 | (48,536 | ) | 5,648 | ||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars in thousands, except share data and per share data)
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | (3,730 | ) | (4,551 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 63 | 32 | ||||||
| Share-based compensation | 229 | 372 | ||||||
| Exchange rate changes in cash and cash equivalents and short time deposits | (452 | ) | (358 | ) | ||||
| Changes in assets and liabilities: | ||||||||
| Decrease (increase) in trade accounts receivable | (77 | ) | 168 | |||||
| Increase in inventory | (386 | ) | (6 | ) | ||||
| Decrease (increase) in prepaid expenses and other receivables | 121 | (253 | ) | |||||
| Decrease (increase) in prepaid expenses and other long-term assets | 17 | (11 | ) | |||||
| Increase in right of use assets | (81 | ) | (225 | ) | ||||
| Increase (decrease) in trade accounts payable | 217 | (28 | ) | |||||
| Increase in lease liabilities | 92 | 240 | ||||||
| Increase (decrease) in other current liabilities | (135 | ) | 2,306 | |||||
| Increase in other long-term liabilities | 432 | 325 | ||||||
| Net cash used in operating activities | (3,690 | ) | (1,989 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Investment of deposits | (4,432 | ) | — | |||||
| Investment of restricted deposits | (15 | ) | — | |||||
| Purchase of property and equipment | (223 | ) | (103 | ) | ||||
| Net cash used in investing activities | (4,670 | ) | (103 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Issuance of ordinary shares, net of issuance expenses | 5,858 | 3,309 | ||||||
| Net cash provided by financing activities | 5,858 | 3,309 | ||||||
| Increase (decrease) in cash and cash equivalents | (2,502 | ) | 1,217 | |||||
| Cash and cash equivalents beginning of the year | 5,789 | 4,214 | ||||||
| Effect of foreign exchange rate on cash and cash equivalents | 215 | 358 | ||||||
| Cash and cash equivalents end of the year | 3,502 | 5,789 | ||||||
F-7
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 1 - GENERAL
| A. | Description of the Company: |
IceCure Medical Ltd. (“IceCure Medical Ltd.”, the “Company”, “we” or “our”) is a medical device company incorporated in Israel.
The Company’s ordinary shares are listed on the Tel Aviv Stock Exchange.
Since its establishment, IceCure Medical Ltd., and its wholly-owned subsidiaries, IceCure Medical Inc. in the United States (the “US Subsidiary”), IceCure Medical HK Limited, in Hong Kong (the “Hong Kong Subsidiary”) and IceCure (Shanghai) MedTech Co., Ltd. In China (the “Chinese Subsidiary, and together with the Company, the US Subsidiary and the Hong Kong Subsidiary, the “Group”), have been engaged in the research, development and commercialization of minimally invasive medical devices for cryoablation (freezing) of tumors in the human body, using its propriety liquid nitrogen Cryoablation technology, as an alternative to surgical intervention to remove the tumor. The Company received regulatory approvals for marketing its products in the United States, Europe and other territories.
The US Subsidiary was established on April 6, 2011 in the State of Delaware and is engaged in business development, marketing, clinical trial management and sale of the Company’s products in the United States. The Hong Kong Subsidiary was established on September 26, 2018 and commenced its activity in 2021. The Chinese Subsidiary was established on July 14, 2020, and is wholly owned by the Hong Kong Subsidiary. The Chinese Subsidiary in China commenced its operation on January 1, 2021 and is engaged in business development and obtaining regulatory approvals for the Company’s products.
The Group activities are subject to significant risks and uncertainties, including failing to secure additional funding to commercialize its technology, obtaining regulatory approvals and other risks. In addition, the Group is subject to risks from, among other things, competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements and limited operating history.
| B. | Risk factors: |
Our ability to operate successfully is materially uncertain and our operations are subject to significant risks inherent in a developing business enterprise.
Additional funding will be required to complete the Company’s research and development and clinical trials, to attain regulatory approvals, to continue our commercialization efforts and to achieve a level of sales adequate to support the Company’s cost structure.
We will need to raise additional funds to meet our anticipated expenses so that we can execute our business plan. We expect to incur substantial and increasing net losses for the foreseeable future as we increase our spending to execute our development programs.
The amount of financing required will depend on many factors including our research and development costs, our clinical trials, our commercialization efforts, and other working capital requirements. Our ability to access the capital markets, or to secure partnerships is mainly dependent on the progress of our research and development, our clinical trials, regulatory approval of our products and our success in commercialization of our products.
F-8
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 1 - GENERAL (Cont.)
| C. | Outbreak of 2019 Novel Coronavirus (“COVID-19”): |
On March 11, 2020, the World Health Organization categorized COVID-19 as a pandemic. As a result, in many countries around the world, including Israel, a series of measures were taken since its outbreak, to contain and reduce the spread of the COVID-19, including, among other things, imposing various restrictions on trading activity, closing borders, restricting gatherings, restricting movement, closing places of employment, etc. It should be noted that the Company, in its capacity as an essential service provider in Israel, in accordance with the addition to the Emergency Regulations (limiting the number of employees in the workplace to reduce the spread of the new COVID-19), has continued its business activities since the outbreak, as well as during closure periods, but with travel restrictions which affected its operations outside of Israel.
Due to the burden on health systems and the fear of infection, medical institutions in certain territories have restricted elective procedures, including those related to the Company’s area of activity, face-to-face meetings, and on-site demonstrations.
However, despite the delays caused in the implementation of management plans in certain territories and fields, such restrictions did not have a material effect on the Company’s revenues for 2020 as presented to and approved by the Company’s Board of Directors. We still experience challenges in 2021, and expect to experience challenges, which are mainly related to delays in shipments of materials by our suppliers, and disruption in access to new medical service providers and costumers. These disruptions might impact our operation and revenue.
The Company examined and continues to examine the consequences of the outbreak, performs risk assessments and implements operational solutions to deal with the crisis. However, the extent of the impact of COVID-19 on the Company’s future results of operations and financial condition, will depend on certain developments, including the duration and spread of the outbreak, both in Israel and globally, all of which are uncertain and cannot be predicted at this point.
| D. | Going Concern: |
As of December 31, 2020, the Company has accumulated losses of $48,536. In the year ended December 31, 2020, the Company generated losses of $3,730 and negative cash flows from operating activities of $3,690.
To date, the management expects the Company to continue to generate substantial operating losses and to continue to fund its operations primarily through utilization of its current financial resources, sales of its products, and through additional raises of capital. On March 9, 2021, according to a share purchase agreement signed on January 27, 2021 and following the approval of Company’s general meeting of shareholders, the Company received $9,000 and issued in return 55,131,333 ordinary shares to four investors. In May 2021, the Company received $6,000 and issued in return 36,754,222 ordinary shares (see note 17A). As of May 20, 2021, the Company’s cash, cash equivalents and short-term deposits were $19,467 thousand.
The management expects that its cash, cash equivalents and short-term deposits as of the issuance date of the financial statements, will be sufficient for 12 months of operation.
The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
F-9
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
| A. | Use of estimates: |
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made. Actual results could differ from those estimates.
| B. | Financial statements in U.S. dollars and functional currency: |
The functional currency of the Company and its subsidiaries is the U.S dollar (“USD” or “dollar” or “$”) since the dollar is the currency of the primary economic environment in which the Company has operated and expects to continue to operate in the foreseeable future. Most of the Company’s revenues are derived from sales outside of Israel, which are based primarily on dollar. In addition, the majority of the Company’s equity raising is denominated in dollars. Thus, the functional currency of the Company and certain subsidiaries is the dollar.
Transactions and balances denominated in dollars are presented at their original amounts. Transactions and balances denominated in foreign currencies have been re-measured to dollars in accordance with the provisions of Accounting Standards Codification (“ASC”) 830-10, “Foreign Currency Translation”.
All transaction gains and losses from re-measurement of monetary balance sheet items denominated in non-dollar currencies are reflected in the statement of operations as financial income or expenses, as appropriate.
| C. | Principles of consolidation: |
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated upon consolidation. Profits from intercompany sales, not yet realized outside the group, were also eliminated.
| D. | Cash and cash equivalents: |
Cash equivalents are short-term highly liquid investments that are readily convertible into cash with original maturities of three months or less. Highly liquid investments with maturities greater than three months are classified as short-term investments.
F-10
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| E. | Inventories: |
Inventories are valued using the lower of cost and net realizable value, and include raw materials, work in progress and finished goods. The cost of inventories is determined as follows:
Cost of raw materials is determined on a standard cost basis utilizing the weighted average cost of historical purchases, which approximates actual cost.
Cost of work in progress (“WIP”) and finished goods are based on the standard cost method and determined on the cost of raw materials and subcontracted work, and the applicable share of the cost of labor on the weighted average cost basis which approximates actual cost.
The Company regularly evaluates the value of inventory based on a combination of factors including the following: historical usage rates, product end of life dates, technological obsolescence and product introductions.
| F. | Property and equipment: |
Property and equipment are stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the related assets. The annual depreciation rates are as follows:
| % | ||
| Machines and equipment | 15 | |
| Computers and software | 33 | |
| Office furniture and equipment | 7 - 15 | |
| Leasehold improvements | Over the shorter of the related lease period or the life of the asset |
The Company periodically performs impairment testing on its long-lived assets either annually or whenever events or changes in circumstances indicate that the carrying amount may not be recoverable.
| G. | Leases: |
On January 1, 2019, the Company early adopted ASU 2016-02, Leases (Topic 842) (“ASU 2016-02”) using the modified retrospective approach for all lease arrangements at the beginning of the period of adoption. Leases existing for the reporting period beginning January 1, 2019 are presented under ASU 2016-02. The Company leases office space and vehicles under operating leases.
Upon adoption, we elected to utilize the package of transition practical expedients, which allowed us to carry forward prior conclusions related to whether any expired or existing contracts are or contain leases, their lease classification and initial direct costs for existing leases. We also made an accounting policy election to not recognize leases with an initial term of twelve months or less within our consolidated balance sheets and to recognize those lease payments on a straight-line basis on our consolidated statements of income over the lease term. We did not have any material short-term leases accounted for under this policy during the years ended December 31, 2020 and December 31,2019.
F-11
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| G. | Leases (Cont.): |
We determine if an arrangement is a lease at inception. Operating lease assets are presented as operating lease right of use (“ROU”) assets, and corresponding operating lease liabilities are presented as lease liabilities within current liabilities (current portions), and as long-term lease liabilities within non - current liabilities (long-term portions), on our consolidated balance sheet.
Operating lease ROU assets and operating lease liabilities are recognized based on the present value of the remaining lease payments over the lease term at commencement date. Our leases do not provide an implicit interest rate. We calculate the incremental borrowing rate to reflect the interest rate that we would have to pay to borrow on a collateralized basis an amount equal to the lease payments in a similar economic environment over a similar term, and consider our historical borrowing activities and market data in this determination. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Our lease terms may include options to extend the lease when it is reasonably certain that we will exercise that option. Lease expense is recognized on a straight-line basis over the lease term.
Some of our leases contain variable lease payments, which are expensed as incurred unless those payments are based on an index or rate. Variable lease payments based on an index or rate are initially measured using the index or rate in effect at lease commencement and included in the measurement of the lease liability; thereafter, changes to lease payments due to rate or index updates are recorded as rent expense in the period incurred. Our lease agreements do not contain any material residual value guarantees or material restrictive covenants. In addition, we do not have any related party leases.
| H. | Contingencies: |
The Company follows ASC 450-20, “Loss Contingencies”, to report accounting for contingencies. Liabilities for loss contingencies arising from claims, assessments, litigation, fines and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. There were no contingencies as of December 31, 2020.
Otherwise, a qualitative disclosure is included in the notes to the financial statements. The loss contingencies are determined by discounting the future cash flows at a pre-tax interest rate, reflecting the current market estimates of the time value of the money and the specific risks of the liability without weighting the Company’s credit risk. The carrying value of the provision is then adjusted in every period so as to reflect the passage of time and the adjustment amount is credited to financial expenses.
F-12
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| I. | Revenue recognition: |
On January 1, 2018, the Company adopted ASC 606 “Revenue from contracts with customers” (“ASC 606”) by using the modified retrospective approach for all contracts not completed as of the date of adoption.
Under ASC 606, revenue is measured as the amount of consideration the Company expects to be entitled to, in exchange for transferring products or providing services to its customers and is recognized when or as performance obligations under the terms of contracts with the Company’s customers are satisfied. ASC 606 prescribes a five-step model for recognizing revenue from contracts with customers: (i) identify contract(s) with the customer; (ii) identify the separate performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the separate performance obligations in the contract; and (v) recognize revenue when (or as) each performance obligation is satisfied.
At contract inception, once the contract is determined to be within the scope of the new revenue standard, the Company assesses whether the goods or services promised within each contract are distinct and, therefore, represent a separate performance obligation. Goods and services that are determined not to be distinct are combined with other promised goods and services. The Company then allocates the transaction price (the amount of consideration the Company expects to be entitled to from a customer in exchange for the promised goods or services) to each performance obligation and recognizes the associated revenue when (or as) each performance obligation is satisfied.
Revenues from product sales are recognized upon the transfer of control, which is generally upon shipment or delivery.
Provisions for discounts, rebates and sales incentives to customers, returns and other adjustments are provided for in the period the related sales are recorded. Sales incentives to customers are not material.
Deferred revenue represents amounts received by the Company for which the related revenues have not been recognized because one or more of the revenue recognition criteria have not been met.
The current portion of deferred revenue represents the amount to be recognized within one year from the balance sheet date based on the estimated performance period of the underlying performance obligation. The noncurrent portion of deferred revenue represents amounts to be recognized after one year through the end of the performance period of the performance obligation. As of December 31, 2020, and 2019, the Company’s deferred revenue balance is $2,111 and $2,152 (out of which $1,376 and $1,045 are presented as current), respectively.
For further analysis of the Company’s main revenue contract, see Note 10 below.
F-13
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| J. | Share-based compensation: |
The Company applies ASC 718, “Share-Based Payment,” which requires the measurement and recognition of compensation expense for all share-based payment awards, including stock options, made to employees and directors under the Company’s stock plans based on estimated fair values.
ASC 718-10 requires companies to estimate the fair value of share-based payment awards on the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s consolidated statement of comprehensive loss In June 2018, the Financial Accounting Standards Board (“FASB”) issued ASU 2018-07, “Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting”, which simplifies the accounting for non-employee share-based payment transactions by aligning the measurement and classification guidance, with certain exceptions, to that for share-based payment awards to employees. The amendments expand the scope of the accounting standard for share-based payment awards to include share-based payment awards granted to non-employees in exchange for goods or services used or consumed in an entity’s own operations and supersedes the guidance related to equity-based payments to non-employees.
The Company elected to early adopt these amendments on January 1, 2019.
Prior to the adoption, the Company accounted for stock options issued to non-employees under ASC 505-50, “Equity: Equity-Based Payments to Non-Employees,” which required the fair value of such non-employee awards to be re-measured at each quarter-end over the vesting period. After the adoption of ASU 2018-07, the accounting guidance is consistent with accounting for employee share-based compensation.
The Company estimates the fair value of stock options granted using a Black-Scholes option-pricing model. The option-pricing model requires a number of assumptions, the most significant of which are the expected stock-price volatility and the expected option term (the time from the grant date until the options are exercised or expire). The Company’s calculations of the expected volatility were based upon actual historical stock-price movements over the period, which was equal to the expected option term. The expected option term was calculated for options granted to employees and directors in accordance with ASC-718-10-S99, using the “simplified” method, and grants to non-employees were based on the contractual term. Historically, the Company has not paid dividends, and has no foreseeable plans to do so. The risk-free interest rate is based on the yield from Israel Treasury zero-coupon bonds with an equivalent term. Changes in the determination of each of the inputs can affect the fair value of the options granted and the results of operations of the Company.
| K. | Research and development costs: |
Research and development costs are charged to the consolidated statement of comprehensive loss as incurred. Grants for funding of approved research and development projects are recognized at the time the Company is entitled to such grants, on the basis of the costs incurred and applied as a deduction from the research and development expenses.
F-14
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| L. | Severance pay: |
Under Israeli employment laws, all of the Company’s employees in Israel are included under Section 14 of the Severance Compensation Act, 1963 (“Section 14”). Pursuant to Section 14, these employees are entitled to monthly deposits at a rate of 8.33% of their monthly salary, made on their behalf by the Company. Payments in accordance with Section 14 exempt the Company from any future severance pay liabilities in respect of those employees. The aforementioned deposits are not recorded as an asset in the Company’s consolidated balance sheets.
| M. | Treasury shares: |
Treasury shares are presented as a reduction of shareholders’ equity, at their cost to the Company, under “Treasury shares”.
| N. | Income taxes: |
The Company accounts for income taxes utilizing the asset and liability method in accordance with ASC 740, “Income Taxes.” Current tax liabilities are recognized for the estimated taxes payable on tax returns for the current year. Deferred tax liabilities or assets are recognized for the estimated future tax effects attributable to temporary differences between the income-tax bases of assets and liabilities and their reported amounts in the consolidated financial statements and for tax loss carry forwards, and are measured using the enacted tax rates and laws, that will be in effect when the differences are expected to reverse. Measurement of current and deferred tax liabilities and assets is based on provisions of enacted tax laws, and deferred tax assets are reduced, if necessary, by the amount of tax benefits, the realization of which is not considered more likely than not based on available evidence. As of December 31, 2020, the Company had a full valuation allowance against deferred tax assets.
ASC 740-10 requires a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement. The Company has not recorded any liability for uncertain tax positions for the year ended December 31, 2020.
| O. | Fair value of financial instruments: |
The Company applies ASC 820, “Fair Value Measurements and Disclosures” (“ASC 820”), pursuant to which fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e., the “exit price”) in an orderly transaction between market participants at the measurement date.
F-15
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| O. | Fair value of financial instruments (Cont.): |
The accounting guidance establishes a three-tiered hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
| ● | Level 1 – Quoted prices in active markets for identical assets or liabilities. | |
| ● | Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and | |
| ● | Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value
The carrying values of cash and cash equivalents, other current assets, other long-term assets, trade accounts payable and other current liabilities approximate their fair value due to the short-term maturity of these instruments.
When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the Company considers
the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when
pricing the asset or liability, such as inherent risk, transfer restrictions and risk of nonperformance.
| P. | Concentrations of credit risk: |
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, deposit and trade accounts receivables.
The majority of the Company’s cash and cash equivalents and deposit are in NIS in a major bank in Israel. The management believes that the financial institutions that hold the Company’s investments are corporations with high credit standing. Accordingly, management believes that low credit risk exists with respect to these financial investments.
The trade accounts receivables of the Company are derived from sales to customers located primarily in the Americas, APAC, and Europe. The Company performs ongoing credit evaluations of its customers’ financial condition. Under certain circumstances, the Company may require advance payments.
| Q. | Segment Reporting: |
The chief operating decision maker (the “CODM”) of the Company is the Chief Executive Officer. The CODM reviews financial information presented on a consolidated basis for purposes of allocating resources and evaluating financial performance. Accordingly, the management has determined that the Company operates in one reportable segment.
F-16
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| R. | Basic and diluted net loss per share: |
Basic net loss per share is computed based on the weighted-average number of ordinary shares outstanding during each year. Diluted net loss per share is computed based on the weighted-average number of ordinary shares outstanding during each year, plus the dilutive potential of the ordinary shares considered outstanding during the year, in accordance with ASC 260-10, “Earnings Per Share”, using the treasury stock method.
All outstanding stock options were excluded from the calculation of the diluted loss per share for the years ended December 31, 2020, and 2019 because all such securities have an anti-dilutive effect.
| S. | Comprehensive loss: |
The purpose of reporting comprehensive income (loss) is to report a measure of all changes in equity of an entity that result from recognized transactions and other economic events of the period resulting from transactions from non-owner sources.
| T. | Recently issued accounting pronouncements: |
From time to time, new accounting pronouncements are issued by FASB, or other standard setting bodies and adopted by the Company as of the specified effective date. Unless otherwise discussed, the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
In December 2019, the FASB issued ASU 2019-12, “Simplifying the Accounting for Income Taxes” (Topic 740) which eliminates the need for an organization to analyze whether the following apply in a given period: (1) exception to the incremental approach for intra-period tax allocation; (2) exceptions to accounting for basis differences when there are ownership changes in foreign investments; and (3) exceptions in interim period income tax accounting for year-to-date losses that exceed anticipated losses. The ASU also is designed to improve financial statement preparers’ application of income tax-related guidance and simplify GAAP for (1) franchise taxes that are partially based on income, (2) transactions with a government that result in a step-up in the tax basis of goodwill, (3) separate financial statements of legal entities that are not subject to tax, and (4) enacted changes in tax laws in interim periods.
This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The Company does not expect that the adoption of this standard will have a material impact on its financial position or results of operations.
F-17
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 3 - DEPOSIT
The amount represents a 6 months deposit bearing annual interest rate of 0.19%.
NOTE 4 - INVENTORY
Composition:
| As of | As of | |||||||
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Raw materials | 380 | 350 | ||||||
| Work in progress | 332 | 252 | ||||||
| Finished goods | 352 | 76 | ||||||
| 1,064 | 678 | |||||||
NOTE 5 - LEASES
On January 1, 2019, the Company early adopted of ASU 2016-02, Leases (Topic 842) (“ASU 2016-02”) using the modified retrospective approach for all lease arrangements at the beginning of the period of adoption. The Company leases office space and vehicles under operating leases.
On January 1, 2020, the Company expended its leases from 494 square meters to approximately 581 square meters at a facility located in Caesarea, Israel, and extended its operating lease agreement for another 12 months until July 14, 2022, with additional option to extend until July 14, 2025. To secure the lease payments, the Company provided a bank guarantee of $20.
In addition, the Company leases vehicles under various operating lease agreements.
As of December 31, 2020, and 2019, total right-of-use assets totaled to approximately $306 and $225 and the lease liabilities for operating leases totaled to approximately $332 and $240, respectively.
Supplemental cash flow information related to operating leases was as follows:
| Year ended December 31, 2020 | Year ended December 31, 2019 | |||||||
| Cash payments for operating leases | 185 | 132 | ||||||
The maturities of operating leases liabilities and the reconciliation of undiscounted cash flows of operating lease to operating lease liabilities as of December 31, 2020 were as follows:
| 2020 | - | 148 | ||||||
| 2021 | 212 | 97 | ||||||
| 2022 | 126 | 15 | ||||||
| 2023 | 23 | - | ||||||
| Undiscounted cash flows of operating leases | 361 | 260 | ||||||
| Less: amount representing interest | (30 | ) | (20 | ) | ||||
| Operating lease liabilities | 331 | 240 |
F-18
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 5 - LEASES (Cont.)
The weighted average lease term and weighted average discount rate as of December 31, 2020 were as follows:
| Year ended December 31, 2020 | Year ended December 31, 2019 | |||||||
| Operating leases weighted average remaining lease term (in years) | 1.48 | 1.7 | ||||||
| Operating leases weighted average discount rate | 8.09 | % | 5.5 | % | ||||
NOTE 6 - PROPERTY AND EQUIPMENT, NET
Composition:
| As of | As of | |||||||
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Cost | ||||||||
| Machines and equipment | 198 | 114 | ||||||
| Computers and software | 142 | 83 | ||||||
| Office furniture and equipment | 65 | 44 | ||||||
| Leasehold improvements | 73 | 14 | ||||||
| 478 | 255 | |||||||
| Less - accumulated depreciation | ||||||||
| Machines and equipment | 62 | 42 | ||||||
| Computers and software | 67 | 35 | ||||||
| Office furniture and equipment | 30 | 26 | ||||||
| Leasehold improvements | 12 | 5 | ||||||
| 171 | 108 | |||||||
| Property and Equipment, Net | 307 | 147 | ||||||
Depreciation and amortization expenses for the years ended December 31, 2020 and 2019 were $63 and $32, respectively.
NOTE 7 - OTHER CURRENT LIABILITIES
Composition:
| As of | As of | |||||||
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Deferred revenues | 1,376 | 2,031 | ||||||
| Provision for royalties | 60 | 32 | ||||||
| Payroll and social benefits | 830 | 503 | ||||||
| Vacation and recuperation provision | 221 | 158 | ||||||
| Accrued expenses and others | 368 | 266 | ||||||
| 2,855 | 2,990 | |||||||
F-19
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 8 - COMMITMENTS AND CONTINGENCIES
| A. | Israeli Innovation Authority (the “IIA”): |
The Company undertook to pay royalties to the IIA in respect of grants received from the IIA for the years 2006 through 2014 for participation in research and development costs. According to the terms of the grants, the IIA was entitled to receive royalties at the rate of 3.5% of the sales, up to the amount of the grants received, including accumulated interest. As of the second half of 2017, new provisions to the grant agreements have entered into force, which stipulate that small companies (up to an annual turnover of $70,000) will pay royalties at the rate of 3%.
The liability to the IIA in Note 6 is calculated based on the Company’s revenue from products developed with grants from the IIA. As of December 31, 2020, based on the second median of 2020 sales, the Company recorded in its books a liability for royalties in an amount of $60.
As of December 31, 2020, the Company has no open application for grants to the IIA.
Total grants received by the Company, including accumulated interest, amounts to approximately $2,455 ($2,186 net of royalties paid), and the entire amount was received prior to 2019. The grants are linked to the exchange rate of the dollar and bears interest of LIBOR per annum.
| B. | Liens: |
The Company pledged a deposit in the amount of NIS 70,000 (approximately $20) to secure a bank guarantee issued in favor of a lease agreement. In addition, the Company pledged a deposit in the amount of $15 in favor of a bank guarantee for a credit card issued. The deposits are presented in the consolidated balance sheets as a non-current asset under “Prepaid expenses and other long-term assets”.
NOTE 9 - SHAREHOLDERS’ EQUITY
| A. | Ordinary shares: |
| (1) | The ordinary shares confer upon the holders the right to receive notice to participate and vote in general meetings of shareholders of the Company, the right to receive dividends, if declared, and the right to participate in the distribution of the surplus assets of the Company in an event of liquidation. |
| (2) | Public placements at Tel Aviv Stock Exchange: |
On February 20, 2019, the Company raised $981 (gross) through a public offering of 4,224,000 ordinary shares at $0.23 per share. After deducting closing costs and fees, the Company received proceeds of approximately $933.
On September 8, 2019, the Company raised $2,430 (gross) through a rights offering of its ordinary shares. According to the rights offering, each shareholder holding 55 ordinary shares of the Company was entitled to purchase one unit comprised of 10 ordinary shares, at a price of $1.68 per unit. Total of 1,441,131 rights comprised of 14,411,308 ordinary shares were issued. After deducting closing costs and fees, the Company received proceeds of approximately $2,375.
On August 5, 2020, the Company raised $6,067 (gross) through a public offering of 41,400,000 ordinary shares at $0.146 per share. After deducting closing costs and fees, the Company received proceeds of approximately $5,847, net of issuance expenses.
| (3) | On September 13, 2020 the Company’s general meeting of shareholders approved the increase of the company’s Authorized share capital to 20,000,000,000 shares and canceled the shares par value. |
F-20
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 9 - SHAREHOLDERS’ EQUITY (Cont.)
| B. | Treasury shares: |
In 2009, the Company has forfeited 43,465 ordinary shares at a value of approximately $41.
| C. | Shares and options to employees: |
| (1) | The fair value of options granted was estimated using the Black-Scholes option pricing model, and based on the following assumptions: |
| For the year ended December 31, | For the year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Exercise price | $0.1 - $1.12 | $0.09 - $1.04 | ||||||
| Expected volatility | 110.4% - 88.77% | 110.4% - 96.33% | ||||||
| Risk-free interest | 2.54% - 0.4% | 2.54% - 1.03% | ||||||
| Expected life of up to (years) | 6.18 | 6.16 | ||||||
| (2) | The following table summarizes the option activity for the year ended December 31, 2020 for options granted to employees, officers and members of the Company’s Board of Directors (“the Board”): |
| Number of Share Options | Weighted Average Exercise Price Per Share | Weighted | ||||||||||
| Balance as of January 1, 2019 | 8,064,008 | $ | 0.19 | |||||||||
| Granted | 2,142,018 | $ | 0.22 | |||||||||
| Expired | (25,106 | ) | $ | 0.27 | ||||||||
| Balance as of December 31, 2019 | 10,180,920 | $ | 0.19 | 8.15 | ||||||||
| Granted | 2,962,807 | $ | 0.20 | |||||||||
| Expired | (286,300 | ) | $ | 0.20 | ||||||||
| Forfeited | (1,101,332 | ) | $ | 0.21 | ||||||||
| Exercised | (110,000 | ) | $ | 0.11 | ||||||||
| Balance as of December 31, 2020 | 11,646,095 | $ | 0.21 | 7.69 | ||||||||
| Exercisable at the end of year | 6,223,021 | $ | 0.21 | 6.79 | ||||||||
As of December 31, 2020, a total of 3,074,627 outstanding and exercisable options are “in the money” with aggregate intrinsic value of $253.
The weighted average fair value of options granted during the year ended December 31, 2020, was $0.15 per share.
F-21
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 9 - SHAREHOLDERS’ EQUITY (Cont.)
| C. | Shares and options to employees: (Cont.) |
| (3) | Options granted during 2019 and 2020: |
| (a) | On March 28, 2019, the Company granted 583,018 options to purchase an aggregate of 583,018 ordinary shares to 5 officers of the Company, as follows: 77,808 options for the Company’s Chief Executive Officer (“CEO”), and 505,210 options for four officers of the Company, at an exercise price of $0.27 per share. The CEO’s options will vest in 16 equal quarterly installments over a period of four years from the date of grant. The officer’s options will vest as follows: a quarter after one year and the rest will vest in 12 equal quarterly installments over a period of three years from March 28, 2020. The options are exercisable for 10 years from the date of grant. |
| (b) | On May 21, 2019, the Company granted 1,559,000 options to purchase an aggregate of 1,559,000 ordinary shares to 24 employees of the Company, as follows: 408,000 options for the Company’s CEO and Chairman of the Board, 384,000 options for three officers of the Company and 767,000 option for 19 employees of the Company, at an exercise price of $0.22 per share. The CEO’s and Chairman of the Board’s options will vest in 16 equal quarterly installments over a period of four years from the date of grant. The officer’s options will vest as follows: a quarter after one year and the rest will vest in 12 equal quarterly installments over a period of three years from May 21, 2020. The 19 employee’s options will vest in four equal installments over a period of four years from the date of grant. The options are exercisable for 10 years from the date of grant. |
| (c) | On June 4, 2020, the Company granted 1,140,746 options to purchase an aggregate of 1,140,746 ordinary shares to 27 employees of the Company, at an exercise price of $0.21 per share. The options will vest in four equal installments over a period of four years from the date of grant. The options are exercisable for 10 years from the date of grant. |
| (d) | On August 30, 2020, the Company granted 1,362,061 options to purchase an aggregate of 1,362,061 ordinary shares to six officers of the Company, as follows: 218,659 options for the Company’s CEO, and 1,143,402 options for five officers of the Company, at an exercise price of $0.19 per share. The CEO’s options will vest in 16 equal quarterly installments over a period of four years from the date of grant. The officer’s options will vest as follows: a quarter after one year and the rest will vest in 12 equally quarterly installments over a period of three years from August 30, 2021. The options are exercisable for 10 years from the date of grant. |
| (e) | On October 28, 2020, the Company granted 460,000 options to purchase an aggregate of 460,000 ordinary shares to 2 officers of the Company, as follows: 260,000 options for the Company’s CEO, and 200,000 options for the Chairman of the Board of Directors, at an exercise price of $0.20 per share. The options will vest as follows: a quarter after one year and the rest will vest in 12 equal quarterly installments over a period of three years from October 28, 2021. The options are exercisable for 10 years from the date of grant. |
F-22
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 9 - SHAREHOLDERS’ EQUITY (Cont.)
| C. | Shares and options to employees: (Cont.) |
| (3) | Option granted during 2019 and 2020: (Cont.) |
| (f) | On December 13, 2020, the Company granted 800,000 options to purchase an aggregate of 800,000 ordinary shares to a Company Board member, at an exercise price of $0.20 per share. The options will vest as follows: |
| a. | 200,000 options - a quarter after one year and the rest will vest in 12 equal quarterly installments over a period of three years from December 13, 2020. |
| b. | 600,000 options- based on target achievement. |
The options are exercisable for 10 years from the date of grant.
| (4) | The total share-based compensation the Company recognized for share-based payments is as follows: |
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Cost of revenues | 16 | 12 | ||||||
| Sales and marketing | 26 | 36 | ||||||
| Research and development | 67 | 151 | ||||||
| General and administrative | 120 | 173 | ||||||
| 229 | 372 | |||||||
As of December 31, 2020, the total unrecognized share-based compensation cost, related to non-vested share option grant arrangements under the plan was $297. This cost is expected to be recognized over the remaining vesting period of four years, until the end of December 31, 2024.
NOTE 10 - REVENUES
The Company’s revenues are derived primarily from the sale of consoles and disposables. Revenues from warranty and services are not material and therefore are included in revenue from consoles in the following table.
Composition:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Consoles | 1,817 | 786 | ||||||
| Disposables | 1,006 | 493 | ||||||
| Exclusive distribution agreement | 1,045 | 348 | ||||||
| 3,868 | 1,627 | |||||||
F-23
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 10 - REVENUES (Cont.)
For maintenance agreements that provide service beyond the Company’s standard warranty and other service agreements, revenue is recognized ratably over the contract term. A time-based measure of progress appropriately reflects the transfer of services to the customer. Payment terms between the Company and its customers vary by the type of customer and the country of sale. The term between invoicing and the payment due date is not significant.
Exclusive distribution agreement
On August 30, 2019, the Company entered into an exclusive distribution agreement with Terumo Corporation (“Terumo”), in which Terumo will be appointed as exclusive distributor of the Company’s products in Japan and in Singapore. According to the agreement, Terumo will take full responsibility for registration, importing, marketing, selling, promoting and distributing the Company’s products for cryoablation of breast cancer in Japan and Singapore.
The agreement is for a period of five years from the date of the receipt of regulatory approvals for the sale of the Company’s products in Japan, which will be extended automatically for an additional period of five years each, unless either party notifies the other party of its intention to terminate the agreement at least one year prior to the end of the period of the agreement (either the initial five year period or any of the renewal periods). The agreement can be canceled in certain circumstances.
Pursuant to the agreement, Terumo will be responsible and will bear the costs of performing the activities that are required, including clinical research, insofar as they may be required, for the purpose of receiving the regulatory approvals in Japan.
As of December 31, 2020, the Company is unable to assess what will be required for the purpose of receiving such regulatory approvals. The Company assesses that, the timeframe for obtaining the regulatory approval in Japan is approximately between three to four years from the time of signing the agreement.
In Singapore, the Company has regulatory approval for the sale of its products.
The Company assessed the following promises in the contract in order to identify all relevant performance obligations:
| ● | Sale of products (consoles and disposables); | |
| ● | Providing technical regulatory and clinical materials and information for obtaining the regulatory approval; | |
| ● | Assistance and support in submitting and obtaining the reimbursement approval from the Japanese Ministry of Health for the medical procedures; | |
| ● | Stand ready obligation to keep providing the consoles and disposables throughout the contract term; and | |
| ● | Providing exclusivity rights to Terumo. |
The Company assessed all of the aforementioned promises and identified 3 performance obligations as follows:
| (1) | Selling products; |
| (2) | Providing technical regulatory and clinical materials and information and support services in obtaining the regulatory approval; and |
| (3) | Assistance and support in submitting and obtaining the reimbursement approval from the Japanese Ministry of Health for the medical procedures. |
The stand ready obligation and the exclusive rights were not recognized as separate performance obligations.
F-24
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 10 - REVENUES (Cont.)
The overall fixed consideration is as follows:
| (1) | $1,000 were paid to the Company for an exclusive distribution rights and sharing of information for the purpose of a submission of a regulatory approval request to the Japanese regulatory authorities; and | |
| (2) | $3,000 were paid to the Company as an advance payment, of which $1,500 were paid in 2019 and the remaining balance was paid in 2020. |
In addition, milestones have been set, for which, if met, the Company will receive the following amounts
(that were identified by the Company as variable amounts):
| (1) | $250 will be paid to the Company upon the submission of an application for regulatory approval for the products in Japan; | |
| (2) | $250 will be paid to the Company upon the receipt of regulatory approval in Japan (considered as variable consideration); and | |
| (3) | $500 will be paid to the Company upon the receipt of approval for medical reimbursement for the procedure in Japan (considered as variable consideration). |
The Company evaluated whether the aforementioned milestones are considered probable of being reached and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal will not occur, the associated milestone value is included in the transaction price. The receipt of the regulatory approval and the receipt of approval for medical reimbursement milestone payments are not within the control of the Company or Terumo, and hence are not considered probable of being achieved until those approvals are received. However, the submission of the application for the regulatory approval milestone payment is considered probable of being achieved and thus the Company included this milestone payment in the allocation of the transaction price.
A total amount of $4,250 was allocated to the identified performance obligations as follows:
| ● | Consoles and disposables. |
| ● | Submission of an application for regulatory approval; and. |
| ● | Assistance in obtaining the regulatory approval. |
The Company recognizes revenues from sales of consoles and disposables when the control is transferred to Terumo and recognizes revenue from assistance in obtaining the regulatory approval over the estimated period as evaluated by the Company.
As of December 31, 2020, the Company has received $4,000 of the total consideration, of which $2,069 was recognized as revenue.
F-25
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
| NOTE | 11 - COST OF REVENUES |
Composition:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Payroll and related benefits (including share-based compensation) | 655 | 473 | ||||||
| Raw materials subcontractors and auxiliary materials | 461 | 389 | ||||||
| Shipping | 39 | 69 | ||||||
| Royalties to the IIA | 116 | 48 | ||||||
| Others | 153 | 124 | ||||||
| 1,424 | 1,103 | |||||||
| NOTE | 12 - RESEARCH AND DEVELOPMENT EXPENSES |
Composition:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Payroll and related benefits (including share-based compensation) | 2,273 | 1,730 | ||||||
| Raw materials subcontracted work and consulting | 1,058 | 866 | ||||||
| Others | 478 | 405 | ||||||
| 3,809 | 3,001 | |||||||
NOTE 13 - SALES AND MARKETING EXPENSES
Composition:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Payroll and related benefits (including share-based compensation) | 576 | 403 | ||||||
| Consulting and professional services | 157 | 150 | ||||||
| Travel | 29 | 167 | ||||||
| Advertising and promotion expenses | 36 | 41 | ||||||
| Sales commissions | 57 | 33 | ||||||
| Others | 208 | 241 | ||||||
| 1,063 | 1,035 | |||||||
F-26
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 14 - GENERAL AND ADMINISTRATIVE EXPENSES
Composition:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Payroll and related benefits (including share-based compensation) | 965 | 833 | ||||||
| Professional services | 611 | 347 | ||||||
| Others | 138 | 92 | ||||||
| 1,714 | 1,272 | |||||||
NOTE 15 - TAXES ON INCOME
| A. | General: |
The Company is assessed for tax purposes on an unconsolidated basis. Each of the Company’s subsidiaries is subject to the tax rules prevailing in its country of incorporation.
| B. | Corporate Taxation: |
The Company is subject to Israeli corporate tax rate of 23% for the years ended December 31, 2020 and December 31, 2019.
The US subsidiary was subject to U.S. tax rate of 21% for the years ended December 31, 2020 and December 31, 2019.
| C. | Net loss carryforward: |
As of December 31, 2020, the Company has an accumulated tax loss carryforward of approximately $52,150, which may be carried forward and offset against taxable income in the future for an indefinite period.
| E. | Tax assessments |
The Company received final tax assessments through the year ended December 31, 2015.
F-27
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 15 - TAXES ON INCOME (Cont.)
| F. | Deferred income taxes: |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
Significant components of the Company’s deferred tax assets are as follows:
| As of December 31, | As of December 31, | |||||||
| 2020 | 2019 | |||||||
| Net loss carryforward | 11,995 | 10,148 | ||||||
| Other reserves and allowance | 51 | 36 | ||||||
| Total deferred tax assets | 12,046 | 10,184 | ||||||
| Valuation allowance | (12,046 | ) | (10,184 | ) | ||||
| Net deferred tax asset | — | — | ||||||
As of December 31, 2020, the Company has provided valuation allowances of $12,046 in respect of deferred tax assets resulting from tax loss carryforward and other temporary differences. The management currently believes that because the Company has a history of losses, it is more likely than not that the deferred tax regarding the loss carryforward and other temporary differences will not be realized in the foreseeable future.
| G. | Effective tax expense (benefit): |
The components of loss before tax and a reconciliation of the Company’s tax expense to the Company’s theoretical statutory tax benefit is as follows:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Loss before taxes: | ||||||||
| Local | 3,735 | 4,555 | ||||||
| Foreign 1 | (5 | ) | (4 | ) | ||||
| Net loss and comprehensive loss, as reported in the consolidated statements of comprehensive loss | 3,730 | 4,551 | ||||||
| Israeli statutory income tax rate | 23 | % | 23 | % | ||||
| Theoretical tax benefit | 858 | 1,047 | ||||||
| Losses and other items for which a valuation allowance was provided or benefit from loss carryforwards | (858 | ) | (1,047 | ) | ||||
| Tax expense | - | - | ||||||
| 1 | Foreign is amount related to the US Subsidiary. |
F-28
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 16 - GEOGRAPHIC AND SIGNIFICANT CUSTOMER INFORMATION
The Company has identified one reportable and operating segment that designs, develops, manufactures and markets Cryoablation medical devices. The results of operations provided to and analyzed by the CODM are at the consolidated level and accordingly, key resources and assessments of performance are performed at the consolidated level. We continue to evaluate our internal reporting structure and the potential impact of any changes on our segment reporting.
The following table sets forth reporting revenue segment information by geographic region:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Israel | 7 | 65 | ||||||
| Japan | 2,097 | 512 | ||||||
| United States | 276 | 231 | ||||||
| China | - | 220 | ||||||
| Spain | 207 | 211 | ||||||
| Thailand | 528 | - | ||||||
| Other 2 | 753 | 388 | ||||||
| 3,868 | 1,627 | |||||||
The following table sets forth reporting property and equipment segment information by geographic region:
| As of December 31, | As of December 31, | |||||||
| 2020 | 2019 | |||||||
| Israel | 305 | 140 | ||||||
| United States | 2 | 7 | ||||||
| 307 | 147 | |||||||
The following table is a summary of customer concentrations as a percentage of total revenue:
| Year ended December 31, | Year ended December 31, | |||||||
| 2020 | 2019 | |||||||
| Customer A | 47 | % | 21 | % | ||||
| Customer B | - | 14 | % | |||||
| Customer C | - | 13 | % | |||||
| Customer D | 14 | % | - | |||||
| 2 | No country represented is greater than 10% of our total revenue as of the dates presented, other than the territories presented above. |
F-29
ICECURE MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share data and per share data)
NOTE 17 - SUBSEQUENT EVENTS
The following are the significant events that took place subsequent to December 31, 2020 through May 24, 2021, which is the date that the accompanying financial statements have been issued:
| A. | On January 27, 2021, the Company entered, subject to the approval of the general meeting of shareholders, into a series of share purchase agreement with its controlling share-holder, Epoch Partner Investments Limited (Epoch), for a total of $7,500, Alpha Capital Anstalt (Alpha), for a total of $4,000, Clover Wolf Capital Limited Partnership (Clover Wolf) for a total of $3,100 and Clover Alpha L.P (Clover Alpha) for a total of $400. According to the agreements, the investors will invest in two tranches a total of $15,000, and in return the Company will issue to the investors a total of 91,885,555 ordinary shares at a share price of $0.163, reflecting a 20% discount on the average closing price of the Company’s share in the seven trading days preceding the date of the transaction’s approval by the Board. The first tranche of $9,000 (60% of the total investment) was received following the approval of the Company’s shareholders at a general meeting of the shareholders, on March 7, 2021 (“the first Closing Date”), and the company issued 55,131,333 shares. The second tranche of $6,000 was to close following the approval of the listing of the Company’s securities on Nasdaq (the “Second Closing Milestone” and such date, “the Second Closing Date”). On May 2021, the Company and the investors agreed to conduct the second tranche prior to the achievement of the Second Closing Milestone, and the second tranche of $6,000 was received on May 9, 2021. Accordingly, the company issued to the investors 36,754,222 shares. |
The Company undertook to take steps to complete the listing of the securities for trading on Nasdaq within a period of 120 days from the first closing date. The Company also granted the investors the right to participate in respect of 50% of any amount of future capital raising that the Company will carry out in the course of 12 months beginning on the Second Closing Date, according to applicable law, and to refrain from issuing other securities for a period of 60 days beginning on the Second Closing Date.
F-30
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors, Officers and Employees
Indemnification
The Israeli Companies Law 5759-2999, or the Companies Law, and the Israeli Securities Law, 5728-1968, or the Securities Law, provide that a company may indemnify an office holder against the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking made in advance of an event or following an event, provided its articles of association include a provision authorizing such indemnification:
| ● | a financial liability imposed on him or her in favor of another person by any judgment concerning an act performed in his or her capacity as an office holder, including a settlement or arbitrator’s award approved by a court; |
| ● | reasonable litigation expenses, including attorneys’ fees, expended by the office holder (a) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (1) no indictment (as defined in the Companies Law) was filed against such office holder as a result of such investigation or proceeding; and (2) no financial liability as a substitute for the criminal proceeding (as defined in the Companies Law) was imposed upon him or her as a result of such investigation or proceeding, or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; or (b) in connection with a monetary sanction; |
| ● | reasonable litigation expenses, including attorneys’ fees, expended by the office holder or imposed on him or her by a court: (1) in proceedings that the company institutes, or that another person institutes on the company’s behalf, against him or her; (2) in a criminal proceedings of which he or she was acquitted; or (3) as a result of a conviction for a crime that does not require proof of criminal intent; and |
| ● | expenses incurred by an office holder in connection with an Administrative Procedure under the Securities Law, including reasonable litigation expenses and reasonable attorneys’ fees. An “Administrative Procedure” is defined as a procedure pursuant to chapters H3 (Monetary Sanction by the Israeli Securities Authority), H4 (Administrative Enforcement Procedures of the Administrative Enforcement Committee) or I1 (Arrangement to prevent Procedures or Interruption of procedures subject to conditions) to the Securities Law. |
The Companies Law also permits a company to undertake in advance to indemnify an office holder, provided that if such indemnification relates to financial liability imposed on him or her, as described above, then the undertaking should be limited and shall detail the following foreseen events and amount or criterion:
| ● | to events that in the opinion of the board of directors can be foreseen based on the company’s activities at the time that the undertaking to indemnify is made; and |
| ● | in amount or criterion determined by the board of directors, at the time of the giving of such undertaking to indemnify, to be reasonable under the circumstances. |
We have entered into indemnification agreements with all of our directors and with all members of our senior management. Each such indemnification agreement provides the office holder with indemnification permitted under applicable law and up to a certain amount, and to the extent that these liabilities are not covered by directors and officers insurance.
II-1
Exculpation
Under the Companies Law, an Israeli company may not exculpate an office holder from liability for a breach of his or her duty of loyalty, but may exculpate in advance an office holder from his or her liability to the company, in whole or in part, for damages caused to the company as a result of a breach of his or her duty of care (other than in relation to distributions), but only if a provision authorizing such exculpation is included in its articles of association. Our amended and restated articles of association provide that we may exculpate, in whole or in part, any office holder from liability to us for damages caused to the company as a result of a breach of his or her duty of care, but prohibit an exculpation from liability arising from a company’s transaction in which our controlling shareholder or officer has a personal interest. Subject to the aforesaid limitations, under the indemnification agreements, we exculpate and release our office holders from any and all liability to us related to any breach by them of their duty of care to us to the fullest extent permitted by law.
Limitations
The Companies Law provides that the Company may not exculpate or indemnify an office holder nor enter into an insurance contract that would provide coverage for any liability incurred as a result of any of the following: (1) a breach by the office holder of his or her duty of loyalty unless (in the case of indemnity or insurance only, but not exculpation) the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice us; (2) a breach by the office holder of his or her duty of care if the breach was carried out intentionally or recklessly (as opposed to merely negligently); (3) any act or omission committed with the intent to derive an illegal personal benefit; or (4) any fine, monetary sanction, penalty or forfeit levied against the office holder.
Under the Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to certain office holders or under certain circumstances, also by the shareholders.
Our amended and restated articles of association permit us to exculpate (subject to the aforesaid limitation), indemnify and insure our office holders to the fullest extent permitted or to be permitted by the Companies Law.
Item 7. Recent Sales of Unregistered Securities
Set forth below are the sales of all securities by the Company since July 2018, which were not registered under the Securities Act. The Company believes that each of such issuances was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, Rule 701 and/or Regulation S under the Securities Act.
On February 20, 2019, we issued 4,224,000 Ordinary Shares pursuant to a public offering on TASE, at a price per share of NIS 0.84 (approximately $0.23). The aggregate net proceeds from the offering were approximately NIS 3,374 thousand (approximately $933 thousand).
From September 8, 2019 to October 7, 2019, we issued 14,411,308 Ordinary Shares in a rights offering on TASE, at a price per share of NIS 0.59 (approximately $0.168). The aggregate net proceeds from the offering were approximately NIS 8,312 thousand (approximately $2,375 thousand).
On August 5, 2020, we issued 41,400,000 Ordinary Shares in a public offering on TASE, at a price per share of NIS 0.5 (approximately $0.146). The aggregate net proceeds from the offering were approximately NIS 19,955 thousand (approximately $5,848 thousand).
On March 10, 2021, we issued 55,131,333 Ordinary Shares, at a price per share of NIS 0.533 (approximately $0.163) for aggregate net proceeds of $9,000 thousand pursuant to the January 2021 SPA.
On May 9, 2021, we issued 36,754,222 Ordinary Shares, at a price per share of NIS 0.533 (approximately $0.163) for aggregate net proceeds of $6,000 thousand pursuant to the January 2021 SPA
Since July 2018, we have granted to our directors, officers, and employees options to purchase an aggregate of 8,860,366 Ordinary Shares under our 2017 Plan, with an exercise prices ranging between $0.18 and $0.69 per share. As of July 6, 2021, 752,656 options granted to directors, officers and employees were exercised, and 1,760,635 options forfeited and expired. The total outstanding amount of options and warrants to directors, officers, employees and consultants as of July 6, 2021 is 11,802,912
II-2
Item 8. Exhibits and Financial Statement Schedules
Exhibits:
| Exhibit Number |
Exhibit Description | |
| 1.1* | Articles of Association of IceCure Medical Ltd. | |
| 4.1* | Opinion of Sullivan & Worcester Tel-Aviv (Har-Even & Co.), Israeli counsel to IceCure Medical Ltd. | |
| 10.1* | Form of Indemnification Agreement. | |
| 10.2* | IceCure Medical Global Equity Plan. | |
| 10.3* | IceCure Medical Compensation Policy. | |
| 10.4* | Securities Purchase Agreement, dated January 26, 2021, by and between IceCure Medical Ltd. and the investors listed therein. | |
| 10.5**^ | Distribution Agreement, dated August 29, 2019, by and between IceCure Medical Ltd. and Terumo Corporation. | |
| 10.6**^ | Distribution Agreement, dated December 31, 2020, by and between IceCure Medical Ltd. and Terumo (Thailand) Company Limited. | |
| 21.1* | List of Subsidiaries. | |
| 23.1* | Consent of Brightman Almagor Zohar & Co., a member firm of Deloitte Touche Tohmatsu, independent registered public accounting firm. | |
| 23.1* | Consent of Sullivan & Worcester Israel (Har-Even & Co.) (included in Exhibit 5.1) | |
| 23.2* | Power of Attorney. |
| * | To be filed by an amendment |
| ** | Filed herewith. |
| ^ | Portions of this exhibit have been omitted under rules of the U.S. Securities and Exchange Commission permitting the confidential treatment of select information. |
Financial Statement Schedules:
All financial statement schedules have been omitted because either they are not required, are not applicable or the information required therein is otherwise set forth in the Company’s financial statements and related notes thereto.
Item 9. Undertakings
(a) The undersigned Registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | To file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Act or Rule 3-19 of this chapter if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the Form F-3. |
| (5) | That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (6) | That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement on Form F-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in on , 2021.
| ICECURE MEDICAL LTD. | ||
| By: | ||
| Eyal Shamir | ||
| Chief Executive Officer | ||
POWER OF ATTORNEY
The undersigned officers and directors of IceCure Medical Ltd. hereby constitute and appoint each of Eyal Shamir and Ronen Tsimerman with full power of substitution, each of them singly our true and lawful attorneys-in-fact and agents to take any actions to enable the Company to comply with the Securities Act, and any rules, regulations and requirements of the SEC, in connection with this registration statement on Form F-1, including the power and authority to sign for us in our names in the capacities indicated below any and all further amendments to this registration statement and any other registration statement filed pursuant to the provisions of Rule 462 under the Securities Act.
Pursuant to the requirements of the Securities Act of 1933, this amendment to the registration statement on Form F-1 has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| Chief Executive Officer, Director | , 2021 | |||
| Eyal Shamir | (Principal Executive Officer) | |||
| Chief Financial Officer, Chief Operations Officer | , 2021 | |||
| Ronen Tsimerman | (Principal Financial and Accounting Officer) | |||
| Director, Chairman of the Board of Directors | , 2021 | |||
| Ron Mayron | ||||
| Director | , 2021 | |||
| Doron Birger | ||||
| Director | , 2021 | |||
| Yang Huang | ||||
| Director | , 2021 | |||
| Oded Tamir | ||||
| Director | , 2021 | |||
| Sharon Levita |
II-5
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, , the duly authorized representative in the United States of IceCure Medical Inc., has signed this registration statement on , 2021.
II-6