
Exhibit 99.1

Corporate Presentation | February 2022 (NASDAQ & TASE: ICCM) icecure - medical.com Freezing cancer in its tracks IceCure Medical enabling non-surgical, treatment of cancerous tumors N as d aq & TA S E : I CC M

Forward Looking Statement N as d aq & TA S E : I CC M 2 Disclaimer: IMPORTANT: The following applies to this document, the oral presentation of the information in this document by IceCure Medical Ltd. (the “Company”, “we” or “us”) and any question and answer session that follows the oral presentation (collectively, the “Presentation”). This Presentation contains express or implied forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other U.S. federal securities laws. For example, we are using forward - looking statements when we discuss our regulatory, marketing and commercialization strategy, the expected timing of obtaining regulatory approval for our various products, patient trials and clinical data readout, proposed trials that may occur in the future, the timing and implementation of our collaborations with various partners and the execution of definitive agreements relating to such collaborations and the potential benefits and impact our products could have on improving patient health care. These forward - looking statements and their implications are based on the current expectations of our management only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward - looking statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products; unforeseen scientific difficulties may develop with our process; our products may be more expensive than we anticipate; results in the laboratory may not translate to equally good results in real clinical settings; our patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products and applications; and loss of market share and pressure on pricing resulting from competition. Except as otherwise required by law, we undertake no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting us, reference is made to our reports filed from time to time with the U.S. Securities and Exchange Commission. This Presentation does not constitute or form part of and should not be construed as an offer or the solicitation of an offer to subscribe for or purchase securities of the Company and nothing contained herein shall form the basis of or be relied on in connection with any contract or commitment whatsoever. No representation, warranty or undertaking, express or implied, is made as to, and no reliance should be placed on, the fairness, accuracy, completeness or correctness of the Presentation . The Presentation has not been independently verified and will not be updated . The Presentation, including but not limited to forward - looking statements, applies only as of the date of this document and is not intended to give any assurances as to future results .

Introducing ProSense® Non - surgical Next - Generation Cryoablation Technology N as d aq & TA S E : I CC M 3 Cryoablation is a minimally invasive image g u i de d ( U S or C T ) t r ea t m en t t h a t u s es e x t r eme c ol d t o f r ee z e a n d ac c u r a t e l y de s t r oy d i s ea s ed t i s s u e w i t h i n t h e t u mor zone IceCure’s flagship product ProSense® c r y oab l a t es t u mo r s q u i c k l y a n d w i t h minimal pain* U t ilizi n g e ff e c t i v e li q u i d n i t r o g e n ( L N 2 ) f o r ma x i m u m f r ee z i n g , s a f e t y a n d e f f i c ac y http://www.youtube.com/watch?v=TfhQJ3SN6wQ * freezing effect on tissue from cryoablation produces less pain compared to heat ablation

Regulatory a pp r ov a l in 14 * countries in cl u d in g U . S . a n d Europe Company Highlights *China – system only †Estimated, according to Grand View Research, Inc. ( www.grandviewresearch.com/industry - analysis/tumor - ablation - market ) Data is for all tumor ablation technologies and indications, including heat ablation Cryoablation, RF, MW and others. The information herein has not been independently verified by the company G r o w in g nu m be r o f global distribution agreements C o lla b o r a t i on wit h A S BrS f o r re g i s t ry t r i a l a nd u p d a t e o f guidelines I C E 3 Br e a s t C anc e r T r i al in U S f o r F DA a pp r ov a l in t r e a t i n g e a r l y - s t a g e breast tumors E x c ell en t P a t i en t & P h ysi c i a n Feedback 28 patents in IP p or t fo l i o for a d v a n ce d LN 2 technology Successful t r a n s i t i on fr om clinical and R&D stages to c o mm e r c ia liz a t i on W i d e m a r k et applications $ 2 . 4 B tu m o r a b l a t ion market by 2026 † C P T cod e for reimbursement of breast cancer cryoablation N as d aq & TA S E : I CC M 4
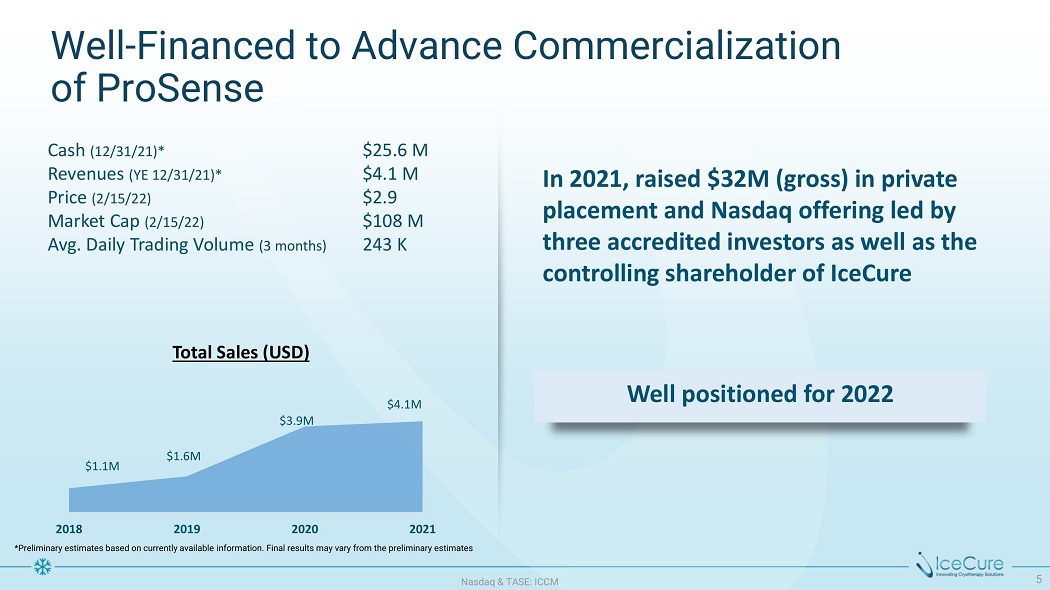
$3 . 9M $1 . 6M $1 . 1M $4 . 1M Well - Financed to Advance Commercialization of ProSense ® In 2021, raised $32M (gross) in private placement and Nasdaq offering led by three accredited investors as well as the controlling shareholder of IceCure N as d aq & TA S E : I CC M 5 Total Sales (USD) Well positioned for 2022 Cash (12/31/21)* Revenues (YE 12/31/21)* Price (2/15/22) Market Cap (2/15/22) Avg. Daily Trading Volume (3 months) $25.6 M $4.1 M $2.9 $108 M 243 K 2018 2019 2020 2021 *Preliminary estimates based on currently available information. Final results may vary from the preliminary estimates
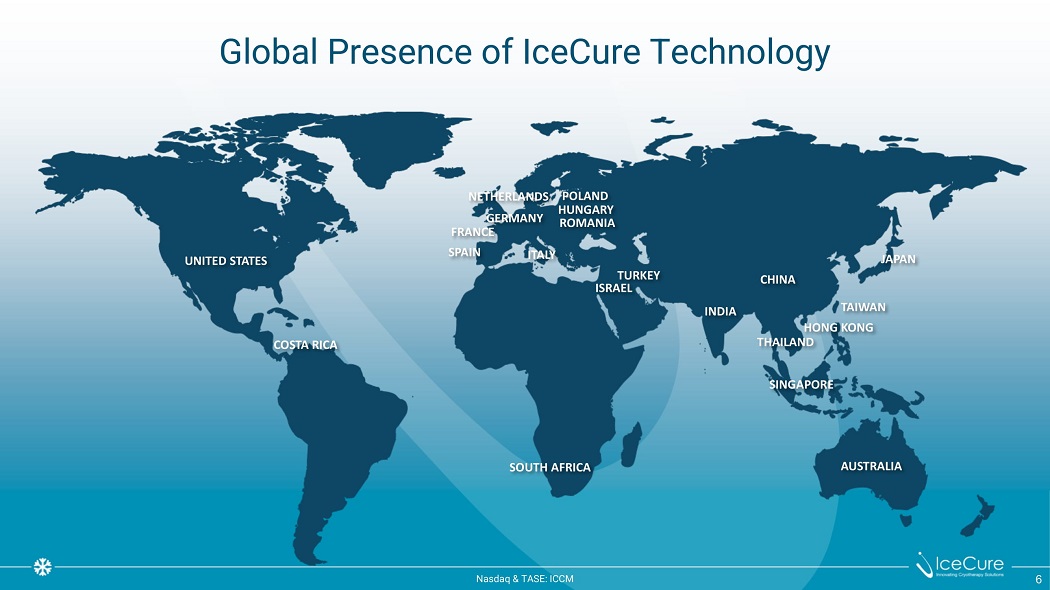
Global Presence of IceCure Technology J A P AN SINGAPORE AUSTRALIA I T A LY S P AIN UNITED STATES C O S T A R ICA SOUTH AFRICA C H INA INDIA T UR KEY ISRAEL T AI W AN HONG KONG THAILAND POLAND HUNGARY ROMANIA NE T H E RL AN D S GERMANY FRANCE N as d aq & TA S E : I CC M 6

T u m o r A b l a tio n Market Opportunities †Estimated, according to Grand View Research, Inc . ( www . grandviewresearch . com/industry - analysis/tumor - ablation - market ) Data is for all tumor ablation technologies and indications, including heat ablation Cryoablation, RF, MW and others . The information herein has not been independently verified by the company K id n e y C an c e r Lung C an c e r C an c e r f u t u r e in d ica t io n s (Such as Prostate Cancer) B on e A ddi t i o n a l L i v e r 118 K N e w ki d n e y an d l i v e r c an c e r p a t i e n t s i n 2021 * Interventional Radiology (CAGR) P o t e n t i a l d r i v en b y n o n / mi n i mal invasive treatments such as Cryoablation† T u m o r A b l a t i o n M a r k et Ex pe c t ed T o R e a c h $2.4 Billion in 2026† Breast Tumors B r e a s t C an c e r Breast Fibroadenoma ~325K new breast cancer patients estimated in 2020* 10% o f f e m al e p o p . e s t . t o h av e f i b r o a d e n o m a s * * * The National Breast Cancer Foundation, Inc. - https://www.nationalbreastcancer.org/wp - content/uploads/2020 - Breast - Cancer - Stats.pdf ** https://www.ncbi.nlm.nih.gov/books/NBK535345/#article - 18600.s6 U S C r y o a b l a tio n G r o wi n g cancer burden I n c r e a s i n g d e m and f o r n o n / m in i m a l - invasive solutions Pu s h f o r r e d uc e d cost of care by insurers and payers $1.0B $2.4B 2018 2026 N as d aq & TA S E : I CC M 7

Regulatory approvals worldwide F D A A pp r o va l f or g en er al minimally - invasive cryoablation applications , specific indications i n c l u d i n g : K i d n ey , L i v e r , N e u r o l o g y , Fibroadenoma F D A B r ea k t h r o u g h D e vi c es Designation for T1 invasive breast c an c e r and / or b re a s t c a n c e r n ot s u i t ab l e for surgical alternatives, prostate, kidney, a n d l i v e r t u mors C E A pp r o va l f o r b e n i g n o r malignant tissue of: B r e a s t , L un g , M u s c u l o s k e l et a l ( b o n e ) , L i v e r & K i d n e y t u mors i n c l . p al l i a t i ve i n t e rve n t i on s R e s t o f W o r ld A pp r o v a l s : I s rae l , S i ng ap or e , H on g - K ong , I n d i a, Thailand, Australia, South Africa, China ( I c e S e n s e 3 S yst e m o n l y ) – s ame clinical indications as CE approval R u ss i a, T a i w a n , C o s t a - R i c a, a n d M e x i c o ( ap p rove d c l i n i c al i n d i c a t i on s may vary) N as d aq & TA S E : I CC M 8

C li n ica l ev id e n ce K i dn e y ca nc e r : I s r a e l I C E S e c r e t T r i a l, 12 0 c a s es I n i t i a l r e s u l t s r ep o r t ed ** 45 small kidney masses (≤ 4 cm) treated i n 4 2 p a t i e n ts a t 1 y e a r f o llo w - u p ( a v e r ag e follow - up period was 18 . 2 months) R e cu rr e nc e f r e e r a t e w a s 93 % One serious adverse event was reported Lung cancer: J ap an I n d ep e n de n t C lin ic a l T r i a l *** P ee r r evi e w ed a r t icle o n 10 1 c a s es Hig h lig h t e d t h a t t h e u s e o f c r yo a b la t io n t r e a t m en t w i t h on l y on e n ee d l e f o r t h e m a j o ri ty o f t h e p a t i e n ts i n t h e tr i a l r e p r e s e n t e d a n advan t ag e i n c o m p a r i s o n t o s y s t e m s t ha t u s e a r g o n g a s , whi c h u s u a ll y r e q ui r e s t h e u s e o f 2 - 3 n ee d l e s f or Musculoskeletal (Bone): Ma in l y p a llia t iv e a n d lo c a l co n t r o l c a s e s in Israel, Italy, France, and Spain 9 Breast cancer: I C E 3 T r i a l 98% recurrence free in the ICE3 trial as of A p r i l 202 1 i n s m a ll , lo w - r i s k , e a r ly - s t ag e malignant breast tumors (190 out of the 19 4 e lig ib l e p a t ie n t s d i d n o t h a v e recurrence) J ap an I n d ep e n de n t T r i a l* 30 4 o f t h e 40 0 p a t i en t s wh o w e r e t r e a t ed w i t h c r yo a b la t io n b e t w ee n 200 6 and 2019; 99% recurrence free rate of breast cancer Fibroadenoma: F i n a li ze d I C E C r y s t a l T r i a l a p r oc e d u r e o n t h e s a m e t u m o r s s i z e *Results reported by Professor E. Fukuma at the International Cryosurgery Society Convention, September 2019 **Presented at the European Association of Urology Conference, March 2019 *** Nomori H, Yamazaki I, Shiraishi A, Adachi T, Kanno M. Cryoablation for T1N0M0 non - small cell lung cancer using liquid nitrogen. Eur J Radiol. 2020;133:109334. doi:10.1016/j.ejrad.2020.109334 N as d aq & TA S E : I CC M

ProSense is Superior to Competing Thermal Ablation Technologies N as d aq & TA S E : I CC M 10 Cryoablation Ic eC u r e P ro S e ns e® T h er m a l A b la t ion (Radiofrequency & Microwave) Pain M i n im a l to n o pa i n * Ve ry p a i n f ul Anesthesia Local H i g h amo unt t o g ener al Visualization Exce ll en t c o n to u r un d er Ultr as o un d & C T L i m i t e d v is ua liz a t i o n Accuracy High Low Imm un e Resp o ns e Positive stimulation Limited Procedure Time 10 – 40 min 10 – 30 min * freezing effect on tissue from cryoablation produces less pain compared to heat ablation
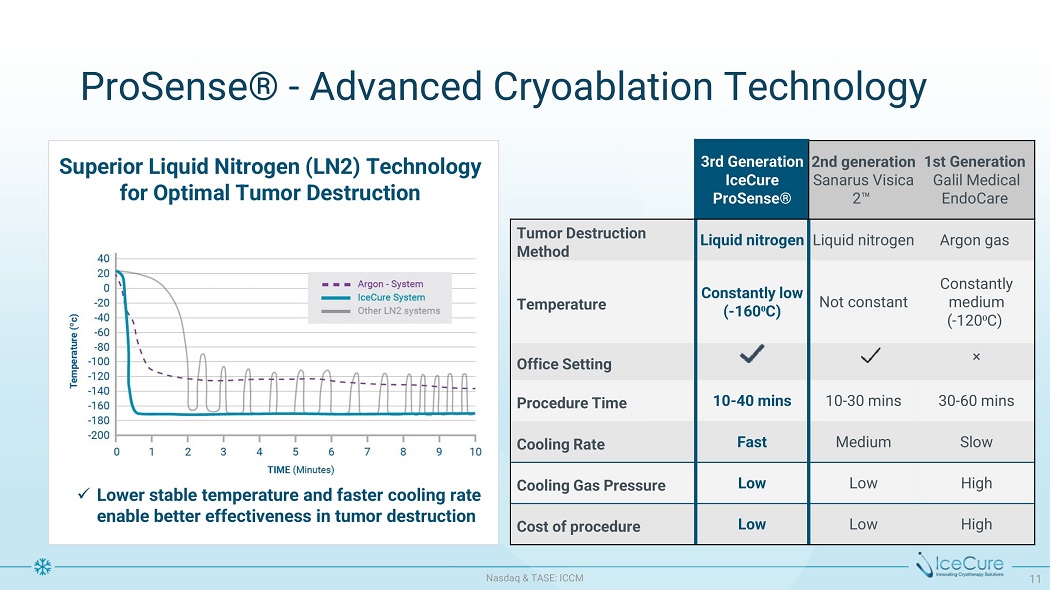
P r oS e n s e ® - A d v a n ce d C r yoa b latio n Tec h n olog y 3 rd G e n e rati o n IceCure ProSense® 2 n d g e n e rati o n S a n ar u s V i s i c a 2 Œ 1s t G e n e ra t i o n Ga li l Me d i c al EndoCare Tu mo r Des t r u c t io n Method Liquid nitrogen L i qu i d n i t rog e n A rgon gas Temperature C o n s t a nt l y l o w ( - 160 ⁰ C) Not constant C on st an t l y medium ( - 120 ⁰ C) Office Se tting ù P r oce d u re T i m e 10 - 40 mins 10 - 30 m i ns 30 - 60 m i ns C ooli ng R a te Fast Medium Slow C ooli ng Gas P r ess u re Low Low High C os t o f pr oce d u re Low Low High Superior Liquid Nitrogen (LN2) Technology f o r O p t i m a l T u m o r D e s t r u c t i on x Low e r s ta b l e t e m p e r a t u r e a nd f a s t e r c ool i n g r a te enable better effectiveness in tumor destruction N as d aq & TA S E : I CC M 11

Breast Tumor Market Activities N as d aq & TA S E : I CC M 12
Challenges in Breast Cancer Surgery (Lumpectomy) • Cost • C o s m e t i c o u t c o m e • 14% of patients undergo re - excision after l u m p ec t o m y d u e t o u n c l ea r m a rg i n s ** • Recovery time • Use of operating rooms places an additional strain on hospital resources ** https://link.springer.com/article/10.1245/s10434 - 019 - 07247 - 5 N as d aq & TA S E : I CC M 13

P r oS e n s e ® - V a lu e f o r A ll *LN 2 , liq u i d n it r o g e n ** h tt p s : // li n k . s p r i n g e r . co m / a r t ic l e / 1 0 . 1 245 / s 10434 - 019 - 072 4 7 - 5 P a ti e n t Ph y s i c i a n Insurer H e a l t h c a r e P r ov i d e r N as d aq & TA S E : I CC M 14 x L N 2 * - M a x i m u m E ff ic a cy x Non - surgical x C os m e t ic a lly S u p e r ior x S a f e r, S i m ple r, F a s t e r & Painless x I mm ed i a t e R e c o ve r y x P r even t i n g R e - e x cis ion A f t e r L u m pe c t o m y fo r B r e a s t Cancer** x E a s y t o Use , I n - o f f ice Procedure x L o w Ri s k , S a f e P r oce d u r e x L N 2 – M a x i m u m E ff ic a cy x F a s t e r - M o r e P a t i e n ts x I n c r e a s ed R O I x L o w e r R ei m b u r s em e n t E x pen s e V s . S u rg e ry x I n - O ffi c e P r o c e d u re x I mm ed i a t e R e c o ve r y x L N 2 – M a x i m u m E ff ic a cy x P a t i e n t D em a n d D r i ves Reimbursement x V a l u e Ba s e d C ar e x P a t i e n t D em a n d D r i ves Reimbursement x F a s t e r , I n - O ffi c e P r o c e d u r e x L o w Ri s k S a f e P r oce d u r e x N o N e w I n f r a s t r u c tu r e x E n v ir o n m e n t a lly & S to r a g e Friendly

Br ea s t C a n ce r & Be n i g n T u mor s U . S . S t r a te g y *BDD is not an FDA Approval, but a designation granted that can expedite the path to marketing clearance for the breast cancer indication **CPT or Current Procedural Terminology is a medical code used by physicians, health insurance companies and accreditation organizations for reimbursement *** The National Breast Cancer Foundation, Inc. - https://www.nationalbreastcancer.org/wp - content/uploads/2020 - Breast - Cancer - Stats.pdf **** https://www.ncbi.nlm.nih.gov/books/NBK535345/#article - 18600 Regulatory Strategy x FDA clearance for general minimally - invasive cryoablation applications x FDA clearance for fibroadenoma (benign breast tumors) cryoablation x Completed ICE3 study enrollment, promising interim results presented at 2021 ASBrS Annual Meeting ; Targeting FDA approval for early - stage and high risk to surgery breast cancer specific cryoablation applications x FDA granted ProSense® Breakthrough Devices Designation* for proposed indications, including for use in the treatment of T 1 invasive breast cancer and/or breast cancer not suitable for surgical alternatives S t ra t eg i c P a r t n er s h i p s x Targeting registry clinical trial with the ASBrS x Targeting ASBrS guidelines amendment following trial results x Collaboration with ASBrS for CPT3** for breast cancer x Targeting CPT1 approval providing reimbursement 325,000 new breast cancer cases estimated in 2020*** Fibroadenoma, Est. 10% of female pop.**** N as d aq & TA S E : I CC M 15

Unique Value Proposition ICE 3 : L an d ma r k U . S . B r e a s t C an c e r T r i al Largest USA controlled multicenter clinical trial ever performed for LN 2 based cryoablation of small, low - risk, early - stage malignant breast tumors as an alternative to surgery “Cryoablation potentially represents a dramatic improvement in care for appropriate low - risk patients, and at three years post - treatment, the ICE3 trial results are extremely positive. The non - invasive procedure is fast, painless and can be delivered under local anesthesia in a doctor’s office. Recovery time is minimal and cosmetic outcomes are excellent with little loss of breast tissue and no scarring. Now, this trial is underscoring the efficacy and safety of the procedure for this patient group.” Interim results presented in April 2021 ASBrS Meeting by ICE3 investigator Richard E Fine, MD, FACS View full ASBrS Press Release View article “Cryoablation Without Excision for Low - Risk, Early - Stage Breast Cancer: 3 - Year Interim Analysis of Ipsilateral Breast Tumor Recurrence in the ICE3 Trial” N as d aq & TA S E : I CC M 16
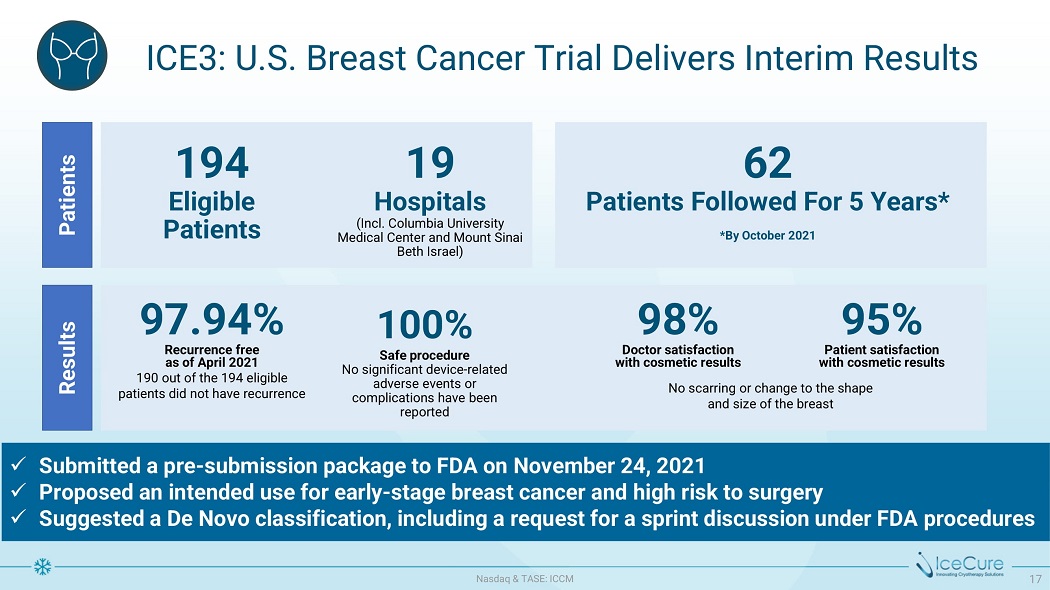
ICE3: U.S. Breast Cancer Trial Delivers Interim Results x Submitted a pre - submission package to FDA on November 24, 2021 x Proposed an intended use for early - stage breast cancer and high risk to surgery x Suggested a De Novo classification, including a request for a sprint discussion under FDA procedures 19 H o s p i t als ( I n cl . C o lu m b i a Un iv e r s i t y Medical Center and Mount Sinai Be t h I s r a e l) Patients Results 194 Eligible Pa t ie n t s 62 P a t i e n t s Fol l ow e d For 5 Y ear s * * By O c t o b er 2021 D o c t o r s a t i s f a c t i on wi t h c osm e t i c r e s u l t s 97.94% R e c u r r e n c e f r ee a s o f A p r i l 2021 19 0 ou t o f t h e 19 4 e lig ib le p a t i e n t s di d n o t h av e r e c u rr e n c e 9 8 % 9 5 % P a t i e n t s a t i s f a c t i on wi t h c osm e t i c r e s u l t s 100% S a f e p r o c e d u r e No significant device - related adverse events or complications have been reported N o s c a rri n g o r c ha n g e t o t h e s ha p e a n d s i z e o f t h e b r e a s t N as d aq & TA S E : I CC M 17
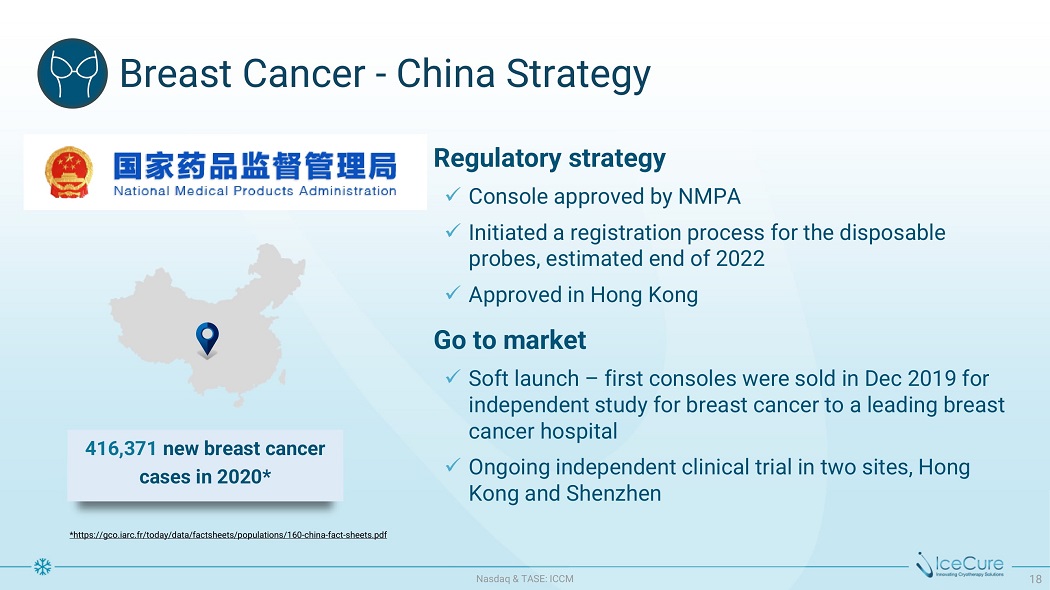
Br ea s t C a n ce r - Ch i n a St r at e g y * https://gco.iarc.fr/today/data/factsheets/populations/160 - china - fact - sheets.pdf Regulatory strategy x C onso l e a pp r ov e d b y N M P A x Initiated a registration process for the disposable p r o b e s , e s t i m a t e d e n d of 2022 x A pp r ov e d i n H on g K ong G o t o m a r k e t x Soft l a u nc h – f i r s t conso l e s w e r e so l d i n D e c 2019 for independent study for breast cancer to a leading breast cancer hospital x Ongoing independent clinical trial in two sites, Hong Kong and Shenzhen 416,371 new breast cancer cases in 2020* N as d aq & TA S E : I CC M 18
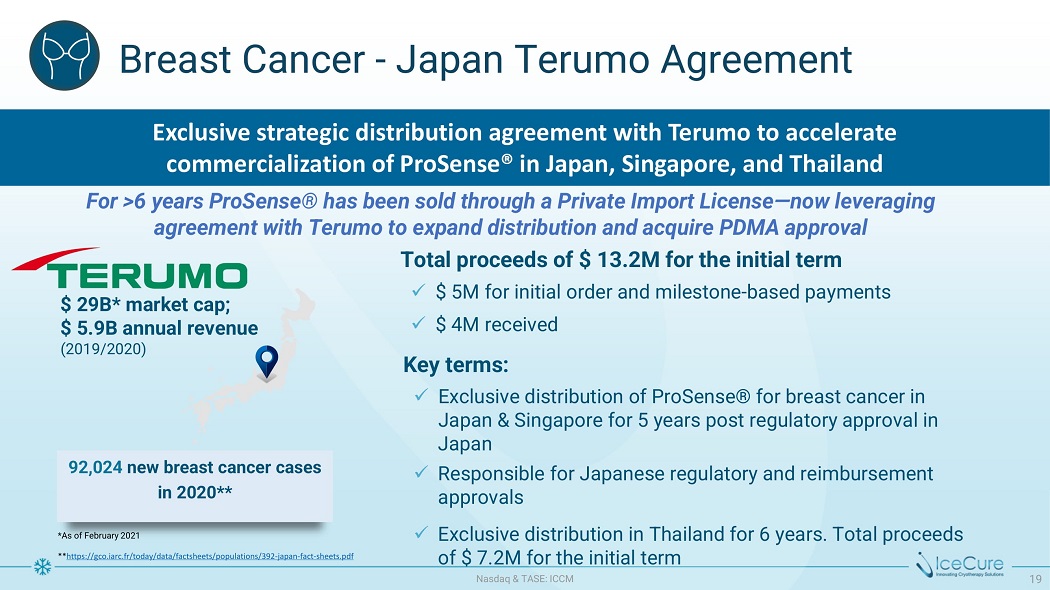
Br ea s t C a n ce r - J a p a n Ter u mo A g r eemen t *A s o f F e b ru a ry 2021 ** https://gco.iarc.fr/today/data/factsheets/populations/392 - japan - fact - sheets.pdf $ 29 B * m a r k e t c a p ; $ 5.9B annual revenue (2019/2020) x Exclusive distribution in Thailand for 6 years. Total proceeds o f $ 7 . 2 M f or t h e i n i t i a l t er m x $ 5M for initial order and milestone - based payments x $ 4M received Ke y t er m s : x Exclusive distribution of ProSense® for breast cancer in Japan & Singapore for 5 years post regulatory approval in Japan x Responsible for Japanese regulatory and reimbursement approvals 92,024 new breast cancer cases in 2020** Exclusive strategic distribution agreement with Terumo to accelerate commercialization of ProSense® in Japan, Singapore, and Thailand For >6 years ProSense® has been sold through a Private Import License — now leveraging a g r e em e n t w i t h T e r u m o t o e x p a n d d i s t r i b u t i o n a n d a cq u ir e PD M A a pp ro v a l T o t a l p r o c ee d s o f $ 13 . 2 M f o r t h e i n i t i a l term N as d aq & TA S E : I CC M 19

F IB RO AD E N O M AS N as d aq & TA S E : I CC M 20
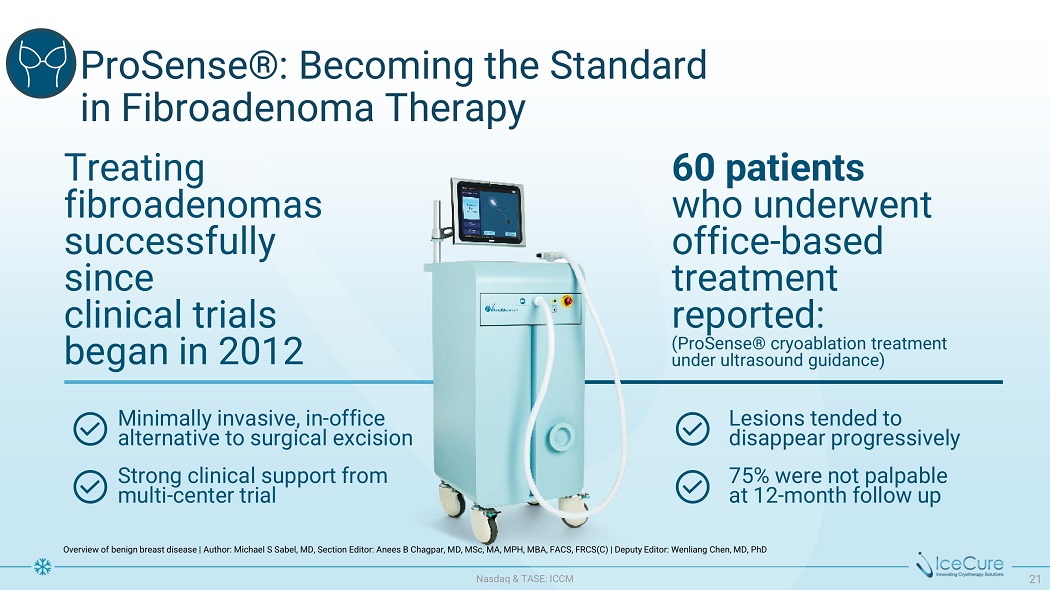
P r oS e n s e ® : Becomi n g th e S ta n d a r d i n Fi b r oa d e n om a T h e r a p y Overview of benign breast disease | Author: Michael S Sabel, MD, Section Editor: Anees B Chagpar, MD, MSc, MA, MPH, MBA, FACS, FRCS(C) | Deputy Editor: Wenliang Chen, MD, PhD Treating f ibro ad e n o ma s successfully since c lin i c al t ria ls be gan i n 2012 60 patients w h o un d e r w e nt office - based treatment reported: (ProSense® cryoablation treatment under ultrasound guidance) M i n im a ll y i n v a s iv e , in - o ff i c e alternative to surgical excision S t r on g c l i n i c a l su pp o r t f r om m u l ti - c e n t e r t r i a l L e s i on s t e nd e d t o disappear progressively 75 % w e r e n o t pa l pab l e at 12 - m on t h fo l l o w u p N as d aq & TA S E : I CC M 21

Kidney C a n c er Lung C a n c er Bone C a n c er Liver C a n c er I n te r ve n tio n a l R a diolog y 22 Na N s a d s a d q a & q T & A T S A E S : E IC : C IC M CM
Interventional Radiology: Expanding Product Line O ve r 500 , 000 n e w p eo p le eac h y ea r ar e d ia g n o s e d w i t h kidney, lung, liver and prostate cancer in the U.S. alone Pain care for bone cancer metastasis • CP T 2 r ei m bu r s e m en t i n t h e U S A • CE approval, actively being used for p r oc e d u r e s i n E U 488 K n e w case s o f k i dn e y cancer globally by 2025* • ICE Secret Trial (Israel) 120 patients ; 141 small kidney masses (≤ 4 cm) treated ; 93 % lack of enhancement on CT or MRI in ( 42 of 45 ) of cases at 1 year follow - up (average follow - up period was 18 . 2 months) • F D A ap p r o va l f o r k i dn ey and liv e r a s o f D e c 2019 • CP T 1 r ei m bu r s e m en t i n t h e US > 2. 5 M n e w case s o f l u n g cancer globally by 2025* • Japan Independent Clinical Trial 101 patients from 2013 – 2019 • Pa t i e n t s wi t h t u mo r s i z e u p t o 1. 2 c m – no r e c u r r e n c e ; Pa t i e n t s wi t h t u mo r s i z e b e t w een 1.3 – 1.7cm, 1 recurrence (4%); Patients with tumor size larger than 1.8 cm – 9 recurrences ( 33 % ) in d ica t in g lo c a l co n t r o l t o b e b e tt e r in smaller tumors (p<0.001) cryoablation t r e a t m en t w i t h on l y on e n ee d l e f o r t h e m a j o r i t y o f p a t i en t s i n t h e t ri a l r ep r e s en t e d a n advan t ag e i n co m p a r i s io n to s y s t e ms t h a t u s e a r g o n g a s , whi c h u s u a ll y r e q ui r e s t h e u s e of 2 – 3 needles for a procedure on the same size *https://gco.iarc.fr/tomorrow/en/dataviz/isotype N as d aq & TA S E : I CC M 23 **Presented at the European Association of Urology Conference, March 2019 *** Nomori H, Yamazaki I, Shiraishi A, Adachi T, Kanno M. Cryoablation for T1N0M0 non - small cell lung cancer using liquid nitrogen. Eur J Radiol. 2020;133:109334. doi:10.1016/j.ejrad.2020.109334

I n te r ve n tio n a l R a diolog y - U S A S t r a te g y *CPT or Current Procedure Terminology is a medical code used by physicians, health insurance companies and accreditation organizations ** A me r i c an C an c e r S o c i e t y .s 6 Regulatory Strategy x FDA approval for general minimally - invasive cryoablation applications x FDA approval for kidney and liver as of Dec 2019 x FDA granted ProSense® Breakthrough Devices Designation for proposed indications, including for use in the treatment of p r o s t a t e , ki dn e y , a n d l i v e r t u m o r s Strategic Partnerships x CPT1* approval and coverage for Cryo treatments of kidney, liver, l un g & b o n e x CPT2 approval and coverage for Cryo treatments of bone cancer 42K Liver cancer patients** 74K Kidney cancer patients** N as d aq & TA S E : I CC M 24
Business Model – Revenue Generators C o n s o le an d c o n s um able p r o b e b u s i n e s s m o d el D i rec t s a l e s a n d v i a d i s t ri bu t ors x D ir e c t s al es t o h o s p i t al s , c l i n i c s a n d doc t or o f f i c es x R e s e lli n g t o d is t r i b u t o r s x Used as a mobile device in different hospitals, clinics, doctor offices in Europe Co n s ol e rel a t ed re v e n u es x Sales of consoles x C on s ol es l oan ed f or a m i n i m u m p u r c h a s e of p r o b es p er mo n t h x Service & maintenance – recurring revenue x Accessories Probes and introducers x Recurring Revenue N as d aq & TA S E : I CC M 25

Proven Leadership Team Shay Levav, VP Clinical, Regulatory & QA Nearly 20 years’ experience in regulatory and quality assurance in the healthcare sector Tlalit Bussi Tel - Tzure, VP Biz Dev & Marketing Over 15 years’ experience in Sales, Biz Dev & Marketing in medical devices Naum Muchnick, VP R&D Over 14 years with GE UltraSound Ron Mayron, Chairman of the Board Served for 20 years in several positions at Teva including as VP – Israel & Africa & CEO of Teva Israel Eyal Shamir, CEO Over 15 years as CEO of medical device companies (B - Cure Laser, Hanita Lenses etc.) Ronen Tsimerman, CFO and COO Over 15 years’ experience as a CFO of public and private companies Merav Nir Dotan, VP Human Resources Over 20 years of experience in human resources and organizational management N as d aq & TA S E : I CC M 26

ProSense® Freezing Cancer In Its Tracks 27 T : +972 - 4 - 623 - 0 333 THANK YOU EYAL Shamir – CEO E: eyals@icecure - medical.com Ronen Tsimerman – CFO/COO E: ronent@icecure - medical.com N as d aq & TA S E : I CC M