
Filed Pursuant to Rule 433
Issuer Free Writing Prospectus
Dated June 25, 2025
Relating to Preliminary Prospectus,
dated June 25, 2025
Registration No. 333-288062

Freezing Cancer in its Tracks Enabling minimally invasive destruction of benign and cancerous tumors with cryoablation (Nasdaq: ICCM) www.icecure - medical.com June 2025 Filed Pursuant to Rule 433 Issuer Free Writing Prospectus Dated June 25, 2025 Relating to Preliminary Prospectus, dated June 16, 2025 Registration No. 333 - 288062

Forward Looking Statement This press release contains forward - looking statements within the meaning of the "safe harbor " provisions of the Private Securities Litigation Reform Act of 1995 and other Federal securities laws . Words such as "expects," "anticipates," "intends," "plans," "believes," "seeks," "estimates" and similar expressions or variations of such words are intended to identify forward - looking statements . For example, IceCure Medical Ltd . (“ IceCure ”, “ IceCure Medical” or the “Company”) is using forward looking statements in this press release when it discusses : the anticipated timing of the FDA decision on the marketing authorization of ProSense ; the Company’s post - market study plan and the prospective approval thereof by the FDA’s Center for Devices and Radiological Health ; that Terumo Corporation is expected to submit its request for breast cancer clearance to the Japanese Pharmaceuticals and Medical Devices Agency in the second half of 2025 ; its cash position ; business, regulatory, marketing and commercialization strategy ; prospective regulatory approvals and the expected timing thereof for its various products worldwide ; that the FDA 510 (k) regulatory clearance for XSense and its commercialization may lead to new uses for certain other clinical indications ; the prospective soft launch of XSense in the first quarter of 2026 ; certain projections in the tumor ablation market ; the expected number of total breast cancer diagnoses in 2025 ; that cryoablation may require further clinical trial studies ; that additional coverage is expected upon the establishment of the permanent CPT Category I code ; that the Company anticipates greater market traction in the rest of the world based on positive U . S . ICE 3 final results ; that more data is expected with ongoing studies of ProSense in 2025 ; that there is a growing number of distribution partnerships with numerous recent regulatory approvals ; and that the potential benefits and impact IceCure’s products could have on improving patient health care . 2

Free Writing Prospectus This presentation highlights basic information about IceCure Medical Ltd. (“IceCure" or the "Company") and the offering to which this presentation relates. As this presentation is a summary, it does not contain all of the information that you should consider before investing in our securities. The Company has filed its Registration Statement on Form F - 1 (File No. 333 - 288062) ("Registration Statement"), including a preliminary prospectus, dated June 25, 2025 (the "Preliminary Prospectus"), with the U.S. Securities and Exchange Commission (the "SEC") for the offering to which this presentation relates. The Registration Statement is not yet effective. Before you invest, you are encouraged to read the Registration Statement, the Preliminary Prospectus, the documents incorporated by reference therein and, when available, the final prospectus, including the risk factors contained or incorporated by reference therein, and other documents that the Company has filed with the SEC for more complete information about the Company and the offering. Prospective investors are able to access these documents for free, including the Preliminary Prospectus, by proceeding to the EDGAR section of the SEC website (www.sec.gov/edgar). Alternatively, the Company and the Dealer - Manager of this offering can arrange to deliver the Preliminary Prospectus and, when available, the final prospectus and/or any supplements thereto by contacting Maxim Group LLC, Prospectus Department, 300 Park Avenue 16th Floor, New York, New York 10022, telephone (212) 895 - 3745 or e - mail: syndicate@maximgrp.com. This presentation does not constitute an offer or invitation for the sale or purchase or to engage in any other transaction with IceCure or its affiliates. The information in this presentation is not targeted at any residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. The offering will only be made by means of a prospectus included in the Registration Statement as and when the Registration Statement is declared effective by the SEC. Any decision to purchase the Company's securities in the proposed offering should be made solely on the basis of the information contained in such prospectus. 3
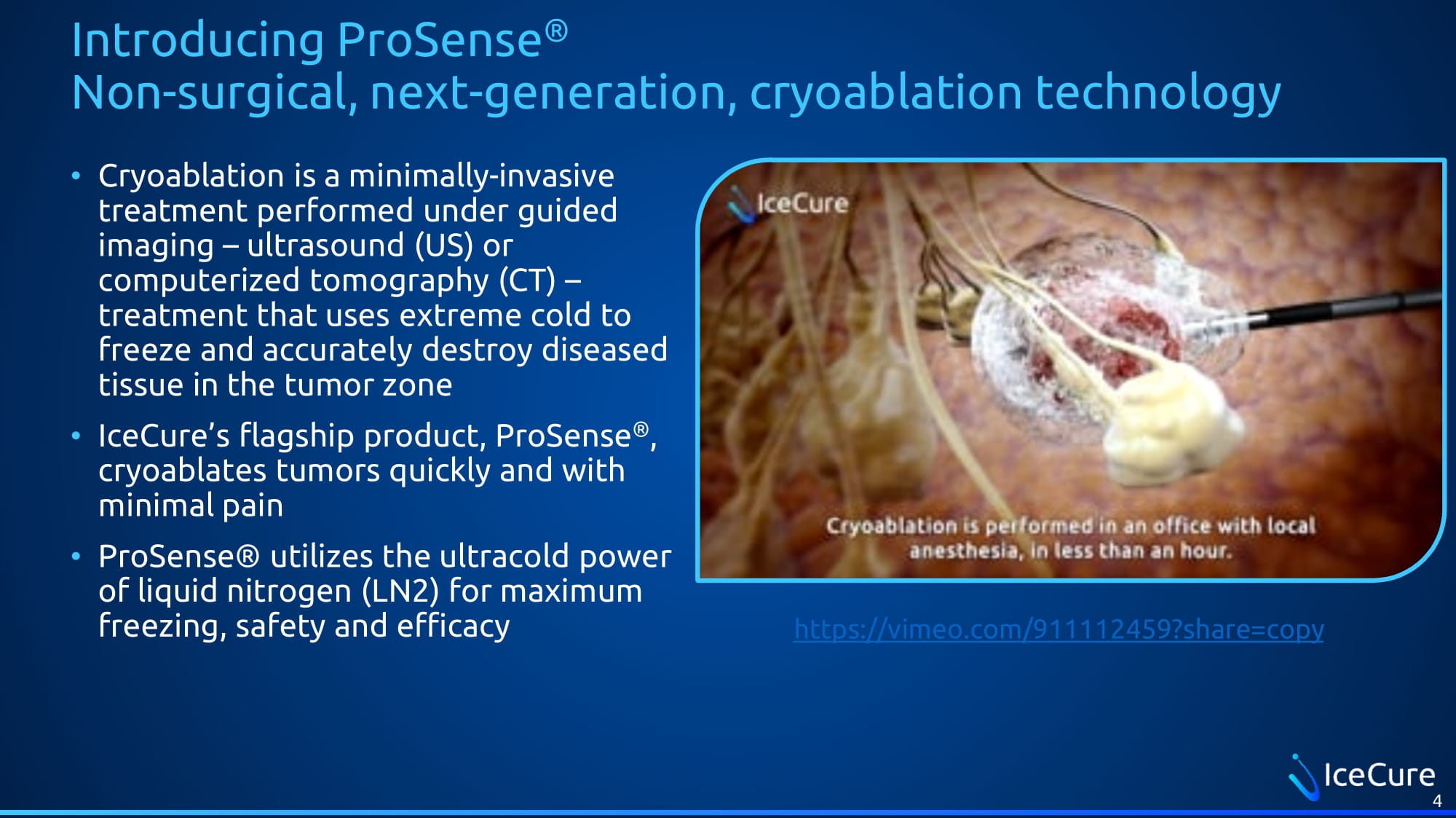
Introducing ProSense ® Non - surgical, next - generation, cryoablation technology • Cryoablation is a minimally - invasive treatment performed under guided imaging – ultrasound (US) or computerized tomography (CT) – treatment that uses extreme cold to freeze and accurately destroy diseased tissue in the tumor zone • IceCure’s flagship product, ProSense ® , cryoablates tumors quickly and with minimal pain • ProSense ® utilizes the ultracold power of liquid nitrogen (LN2) for maximum freezing, safety and efficacy https://vimeo.com/911112459?share=copy 4
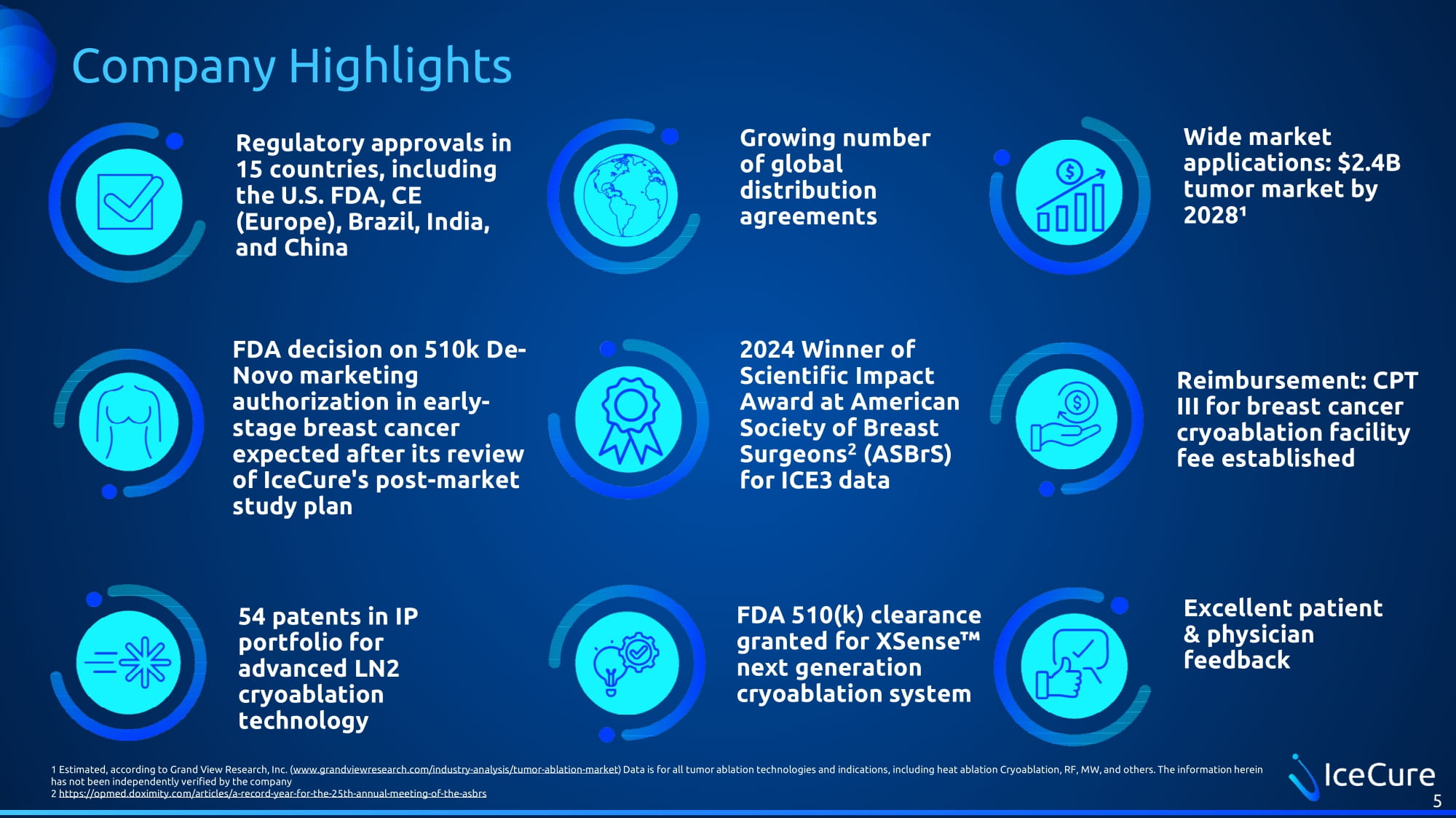
Company Highlights Regulatory approvals in 15 countries, including the U.S. FDA, CE (Europe), Brazil, India, and China 5 Growing number of global distribution agreements Wide market applications: $2.4B tumor market by 2028¹ FDA decision on 510k De - Novo marketing authorization in early - stage breast cancer expected after its review of IceCure's post - market study plan 2024 Winner of Scientific Impact Award at American Society of Breast Surgeons 2 (ASBrS) for ICE3 data Reimbursement: CPT III for breast cancer cryoablation facility fee established 54 patents in IP portfolio for advanced LN2 cryoablation technology FDA 510(k) clearance granted for XSense next generation cryoablation system Excellent patient & physician feedback 1 Estimated, according to Grand View Research, Inc. ( www.grandviewresearch.com/industry - analysis/tumor - ablation - market ) Data is for all tumor ablation technologies and indications, including heat ablation Cryoablation, RF, MW, and others. The inf ormation herein has not been independently verified by the company 2 https://opmed.doximity.com/articles/a - record - year - for - the - 25th - annual - meeting - of - the - asbrs
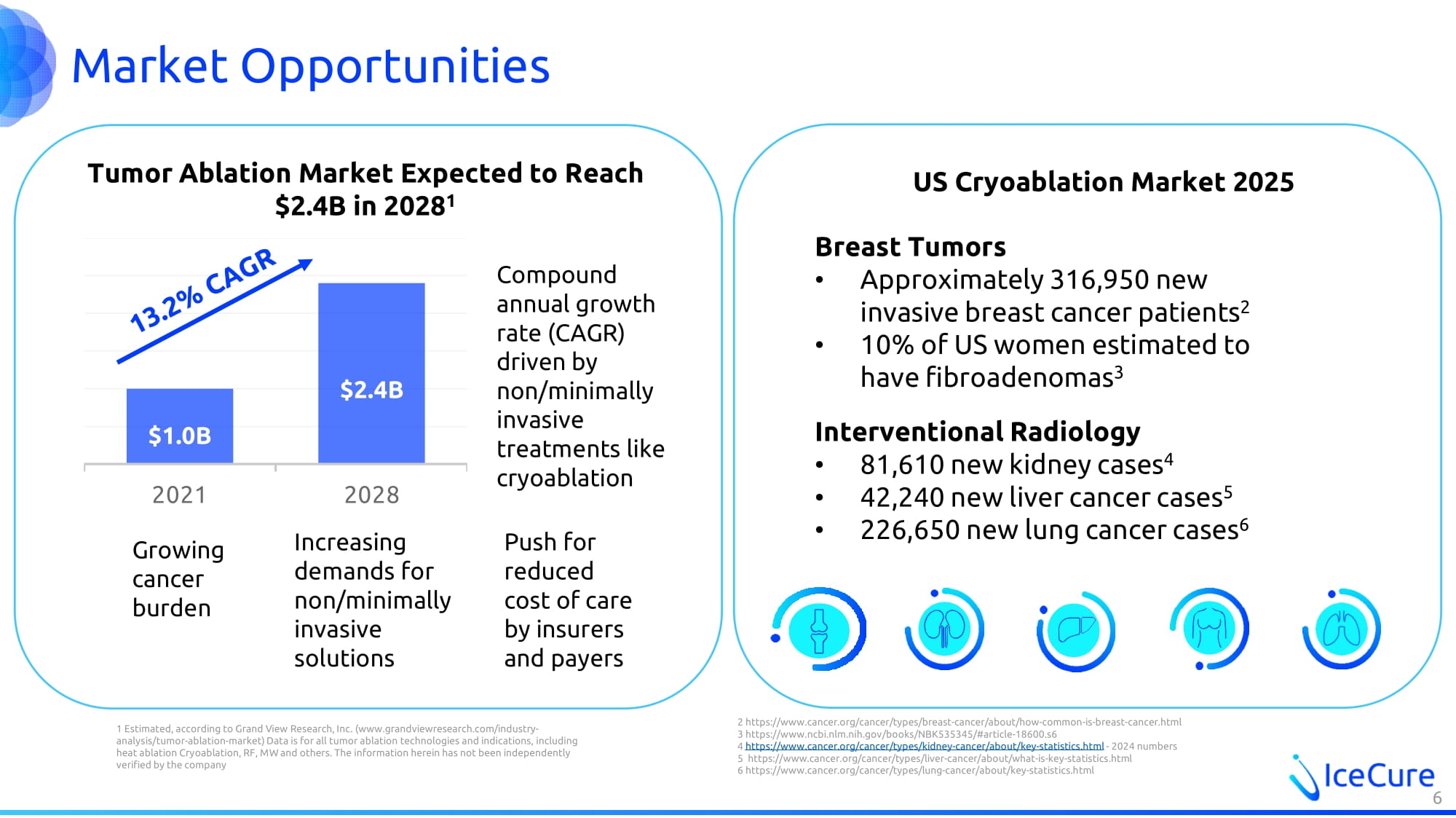
Market Opportunities 1 Estimated, according to Grand View Research, Inc. (www.grandviewresearch.com/industry - analysis/tumor - ablation - market) Data is for all tumor ablation technologies and indications, including heat ablation Cryoablation, RF, MW and others. The information herein has not been independently verified by the company 6 Tumor Ablation Market Expected to Reach $2.4B in 2028 1 US Cryoablation Market 2025 Breast Tumors • Approximately 316,950 new invasive breast cancer patients 2 • 10% of US women estimated to have fibroadenomas 3 Interventional Radiology • 81,610 new kidney cases 4 • 42,240 new liver cancer cases 5 • 226,650 new lung cancer cases 6 2 https://www.cancer.org/cancer/types/breast - cancer/about/how - common - is - breast - cancer.html 3 https://www.ncbi.nlm.nih.gov/books/NBK535345/#article - 18600.s6 4 https://www.cancer.org/cancer/types/kidney - cancer/about/key - statistics.html - 2024 numbers 5 https://www.cancer.org/cancer/types/liver - cancer/about/what - is - key - statistics.html 6 https://www.cancer.org/cancer/types/lung - cancer/about/key - statistics.html $1.0B $2.4B 2021 2028 Compound annual growth rate (CAGR) driven by non/minimally invasive treatments like cryoablation Growing cancer burden Increasing demands for non/minimally invasive solutions Push for reduced cost of care by insurers and payers

Regulatory Approvals Worldwide Rest of the world approvals : India, Thailand, Israel, Brazil, Canada, Singapore, Hong Kong, Australia, and South Africa have similar clinical indications as CE approval for ProSense®. Russia, Taiwan, Costa Rica, and Mexico have ProSense approvals although clinical indications vary by country. • FDA Clearance for general minimally - invasive cryoablation applications with specific indications including: kidney, liver, neurology, fibroadenoma • FDA Marketing Authorization decision expected for early - stage, low - risk T1 invasive breast cancer in patients aged 70+ with adjuvant endocrine therapy following the FDA's review of IceCure's post - market study plan; An FDA Advisory Panel in November 2024 voted favorably of ProSense ®'s benefit - risk profile 7 • CE mark for benign or malignant tissue of the breast, lung, liver, kidney, musculoskeletal (bone), including palliative interventions • NMPA approval in China for ICESense3 System and disposable cryoprobes; clinical indications similar to CE approval
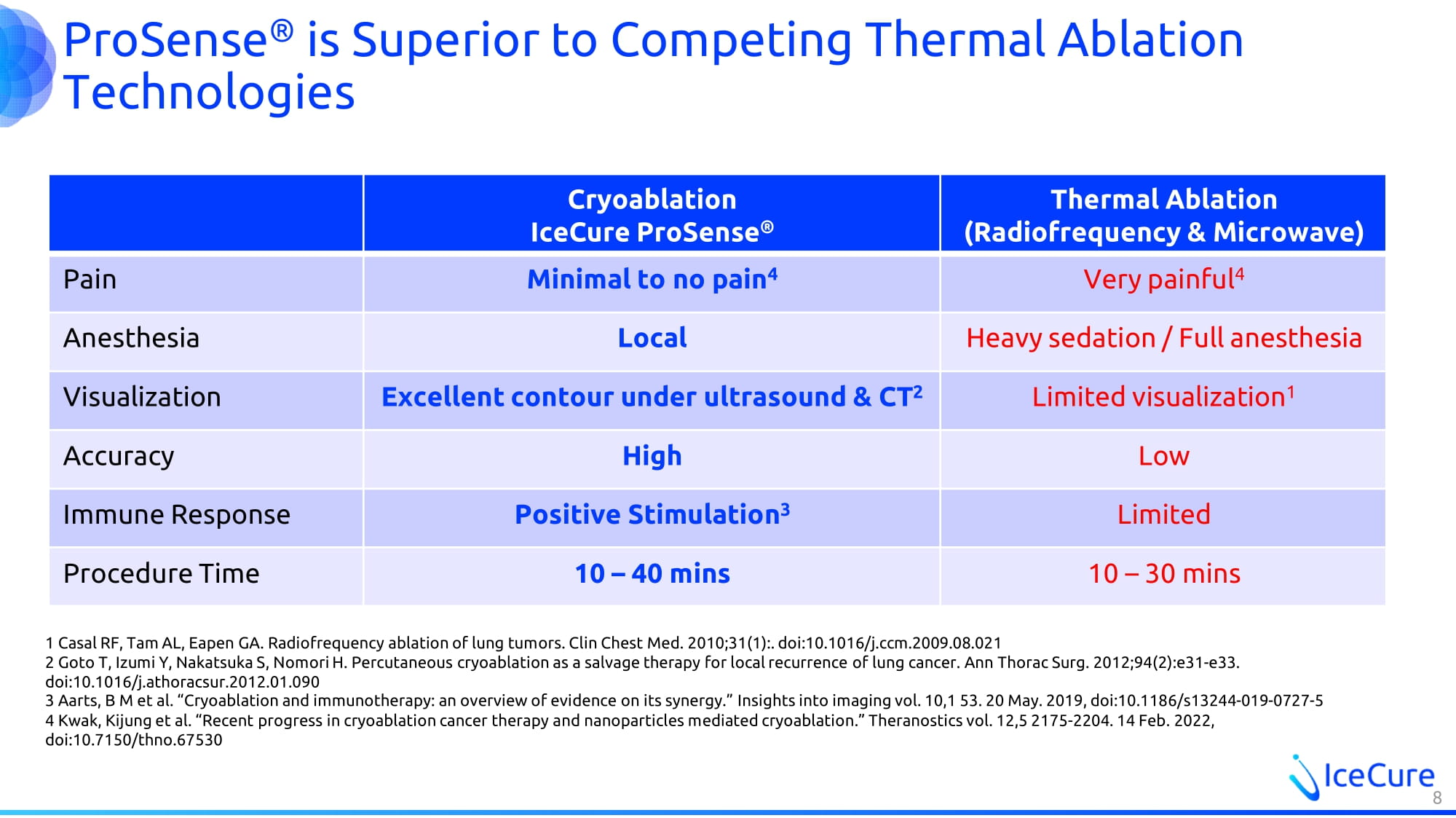
ProSense ® is Superior to Competing Thermal Ablation Technologies Thermal Ablation (Radiofrequency & Microwave) Cryoablation IceCure ProSense ® Very painful 4 Minimal to no pain 4 Pain Heavy sedation / Full anesthesia Local Anesthesia Limited visualization 1 Excellent contour under ultrasound & CT 2 Visualization Low High Accuracy Limited Positive Stimulation 3 Immune Response 10 – 30 mins 10 – 40 mins Procedure Time 8 1 Casal RF, Tam AL, Eapen GA. Radiofrequency ablation of lung tumors . Clin Chest Med. 2010;31(1):. doi:10.1016/j.ccm.2009.08.021 2 Goto T, Izumi Y, Nakatsuka S, Nomori H. Percutaneous cryoablation as a salvage therapy for local recurrence of lung cancer. Ann Thorac Surg. 2012;94(2):e31 - e33. doi:10.1016/j.athoracsur.2012.01.090 3 Aarts, B M et al. “Cryoablation and immunotherapy: an overview of evidence on its synergy.” Insights into imaging vol. 10,1 5 3. 20 May. 2019, doi:10.1186/s13244 - 019 - 0727 - 5 4 Kwak, Kijung et al. “Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation.” Theranostics vol. 12,5 2175 - 2204. 14 Feb. 2022, doi:10.7150/thno.67530

9 Breast Tumor Market Activities
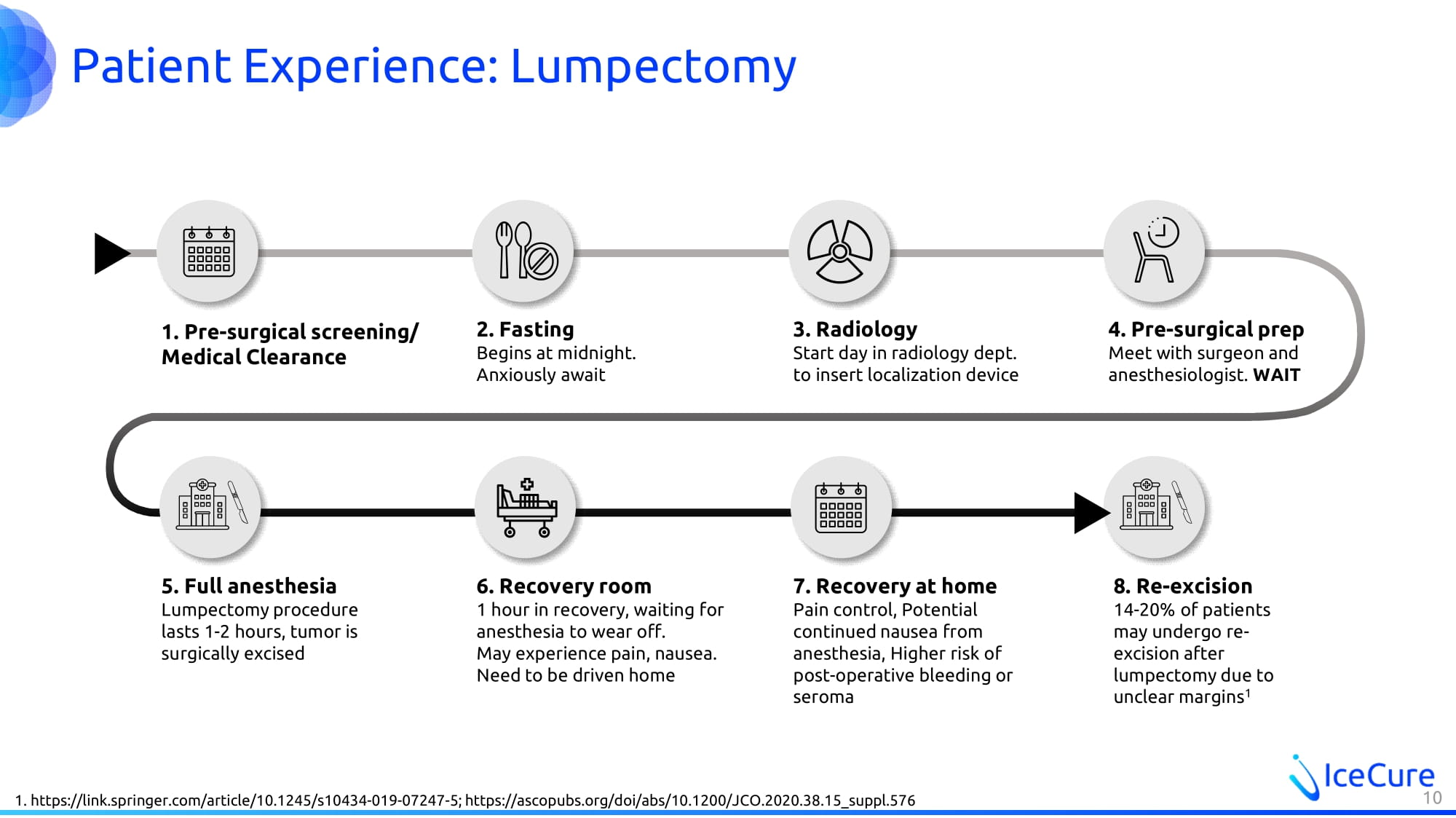
Patient Experience: Lumpectomy 1. Pre - surgical screening/ Medical Clearance 7. Recovery at home Pain control, Potential continued nausea from anesthesia, Higher risk of post - operative bleeding or seroma 2. Fasting Begins at midnight. Anxiously await 3. Radiology Start day in radiology dept. to insert localization device 4. Pre - surgical prep Meet with surgeon and anesthesiologist. WAIT 5. Full anesthesia Lumpectomy procedure lasts 1 - 2 hours, tumor is surgically excised 6. Recovery room 1 hour in recovery, waiting for anesthesia to wear off. May experience pain, nausea. Need to be driven home 8. Re - excision 14 - 20% of patients may undergo re - excision after lumpectomy due to unclear margins 1 1. https://link.springer.com/article/10.1245/s10434 - 019 - 07247 - 5; https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.576 10
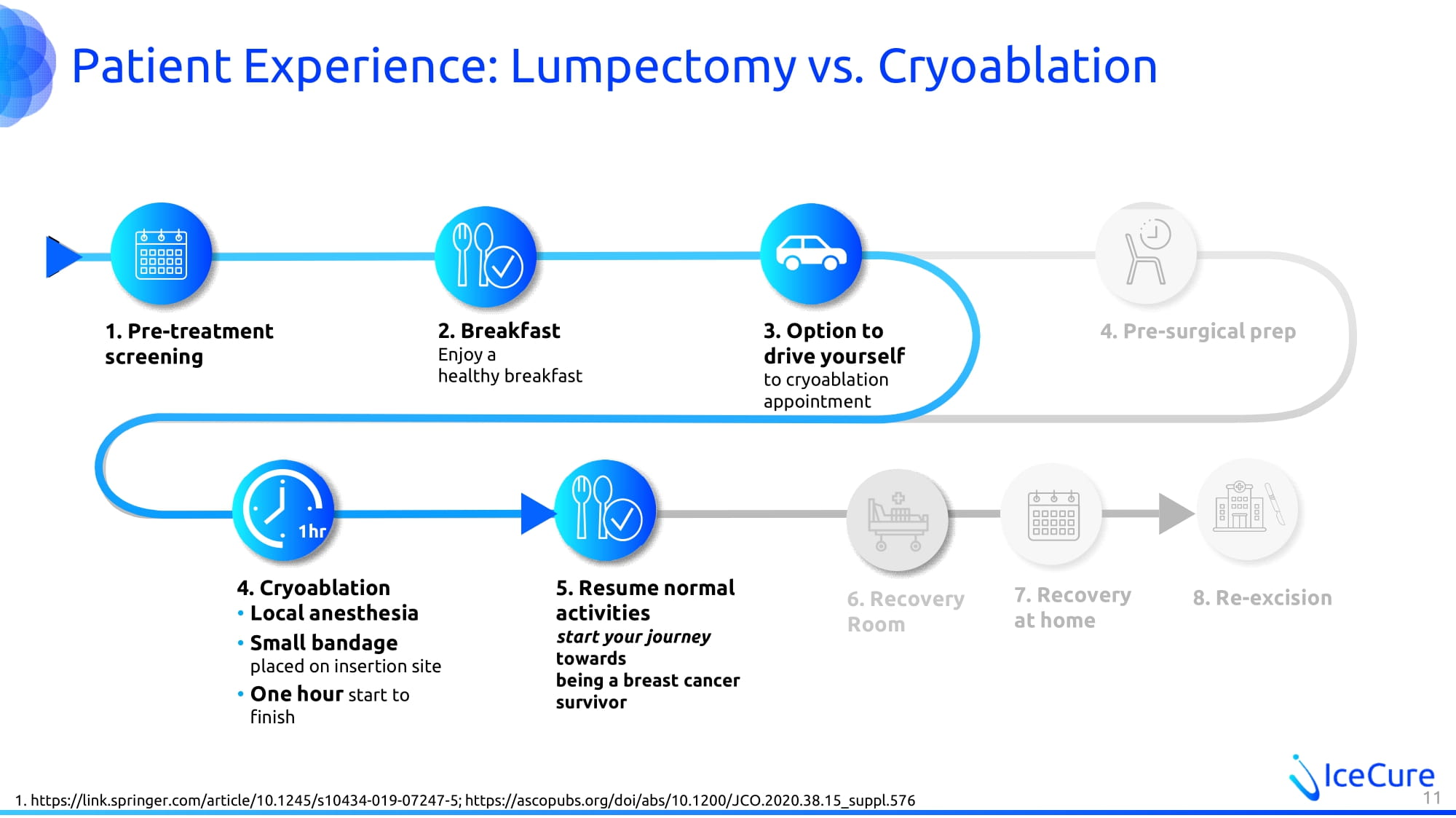
Patient Experience: Lumpectomy vs. Cryoablation 1. https://link.springer.com/article/10.1245/s10434 - 019 - 07247 - 5; https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.576 7. Recovery at home 4. Pre - surgical prep 8. Re - excision 2. Breakfast Enjoy a healthy breakfast 3. Option to drive yourself to cryoablation appointment 4. Cryoablation • Local anesthesia • Small bandage placed on insertion site • One hour start to finish 5. Resume normal activities start your journey towards being a breast cancer survivor 1hr 1. Pre - treatment screening 11 6 . Recovery Room
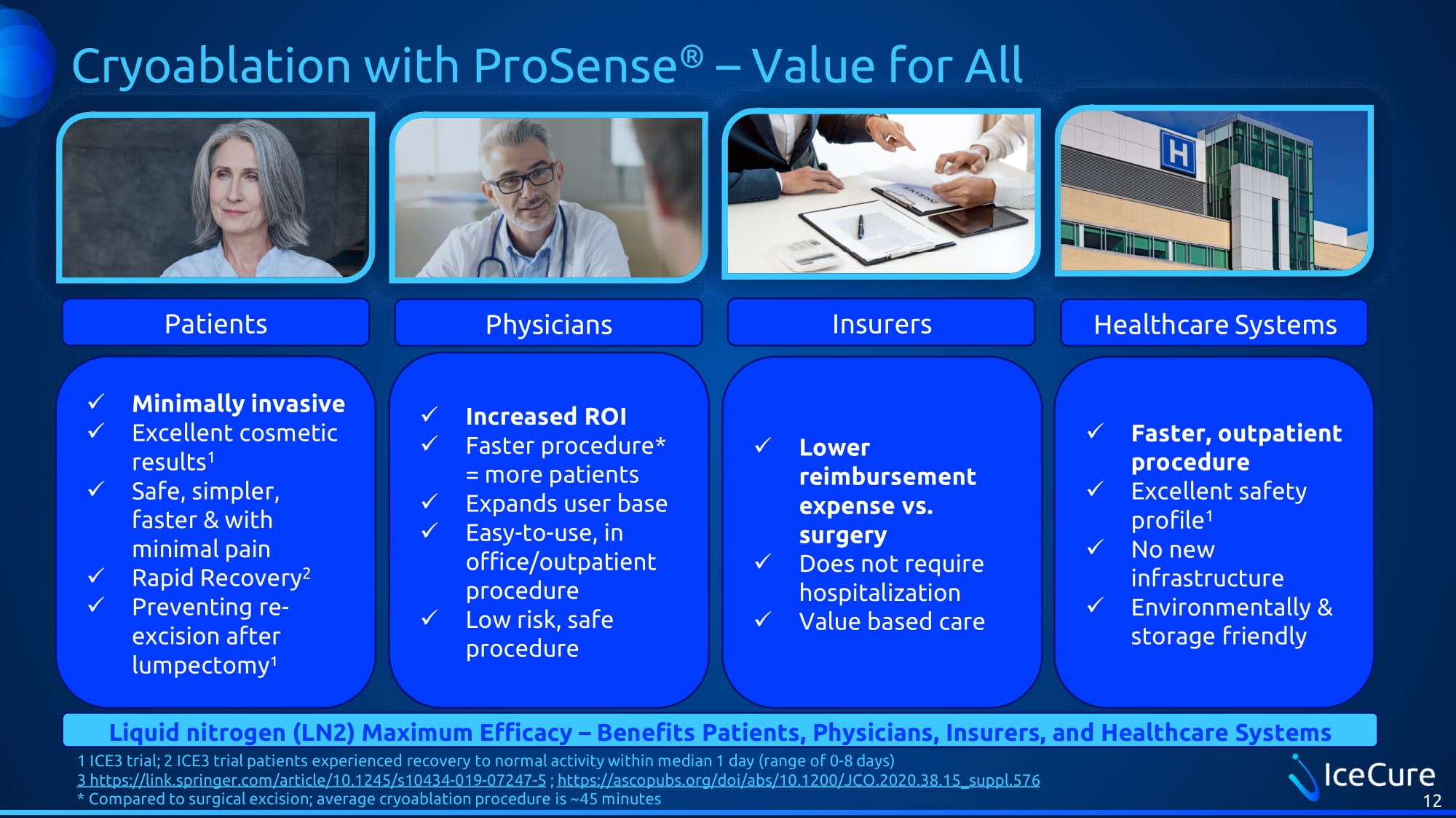
Cryoablation with ProSense ® – Value for All x Minimally invasive x Excellent cosmetic results 1 x Safe, simpler, faster & with minimal pain x Rapid Recovery 2 x Preventing re - excision after lumpectomy¹ x Increased ROI x Faster procedure* = more patients x Expands user base x Easy - to - use, in office/outpatient procedure x Low risk, safe procedure x Faster, outpatient procedure x Excellent safety profile 1 x No new infrastructure x Environmentally & storage friendly 12 x Lower reimbursement expense vs. surgery x Does not require hospitalization x Value based care Patients Physicians Insurers Healthcare Systems Liquid nitrogen (LN2) Maximum Efficacy – Benefits Patients, Physicians, Insurers, and Healthcare Systems 1 ICE3 trial; 2 ICE3 trial patients experienced recovery to normal activity within median 1 day (range of 0 - 8 days) 3 https://link.springer.com/article/10.1245/s10434 - 019 - 07247 - 5 ; https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.576 * Compared to surgical excision; average cryoablation procedure is ~45 minutes
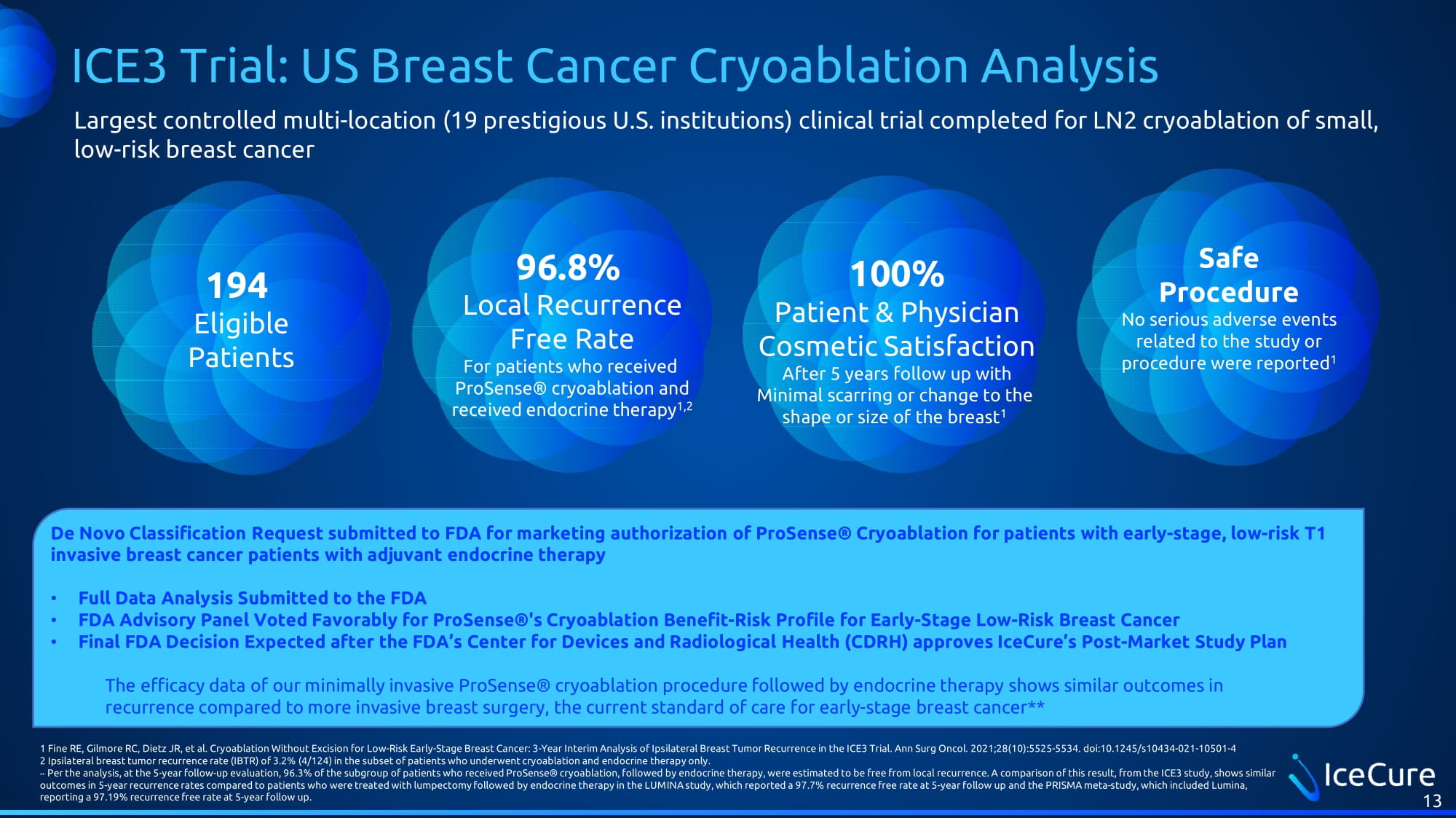
ICE3 Trial: US Breast Cancer Cryoablation Analysis 13 194 Eligible Patients 96.8% Local Recurrence Free Rate For patients who received ProSense® cryoablation and received endocrine therapy 1,2 Safe Procedure No serious adverse events related to the study or procedure were reported 1 100% Patient & Physician Cosmetic Satisfaction After 5 years follow up with Minimal scarring or change to the shape or size of the breast 1 De Novo Classification Request submitted to FDA for marketing authorization of ProSense ® Cryoablation for patients with early - stage, low - risk T1 invasive breast cancer patients with adjuvant endocrine therapy • Full Data Analysis Submitted to the FDA • FDA Advisory Panel Voted Favorably for ProSense ®'s Cryoablation Benefit - Risk Profile for Early - Stage Low - Risk Breast Cancer • Final FDA Decision Expected after the FDA’s Center for Devices and Radiological Health (CDRH) approves IceCure’s Post - Market Study Plan The efficacy data of our minimally invasive ProSense ® cryoablation procedure followed by endocrine therapy shows similar outcomes in recurrence compared to more invasive breast surgery, the current standard of care for early - stage breast cancer** 1 Fine RE, Gilmore RC, Dietz JR, et al. Cryoablation Without Excision for Low - Risk Early - Stage Breast Cancer: 3 - Year Interim Ana lysis of Ipsilateral Breast Tumor Recurrence in the ICE3 Trial. Ann Surg Oncol. 2021;28(10):5525 - 5534. doi:10.1245/s10434 - 021 - 10501 - 4 2 Ipsilateral breast tumor recurrence rate (IBTR) of 3.2% (4/124) in the subset of patients who underwent cryoablation and en doc rine therapy only. ** Per the analysis, at the 5 - year follow - up evaluation, 96.3% of the subgroup of patients who received ProSense ® cryoablation, followed by endocrine therapy, were estimated to be free from local recurrence. A comparison of this result, fro m the ICE3 study, shows similar outcomes in 5 - year recurrence rates compared to patients who were treated with lumpectomy followed by endocrine therapy in the L UMINA study, which reported a 97.7% recurrence free rate at 5 - year follow up and the PRISMA meta - study, which included Lumina, reporting a 97.19% recurrence free rate at 5 - year follow up. Largest controlled multi - location (19 prestigious U.S. institutions) clinical trial completed for LN2 cryoablation of small, low - risk breast cancer
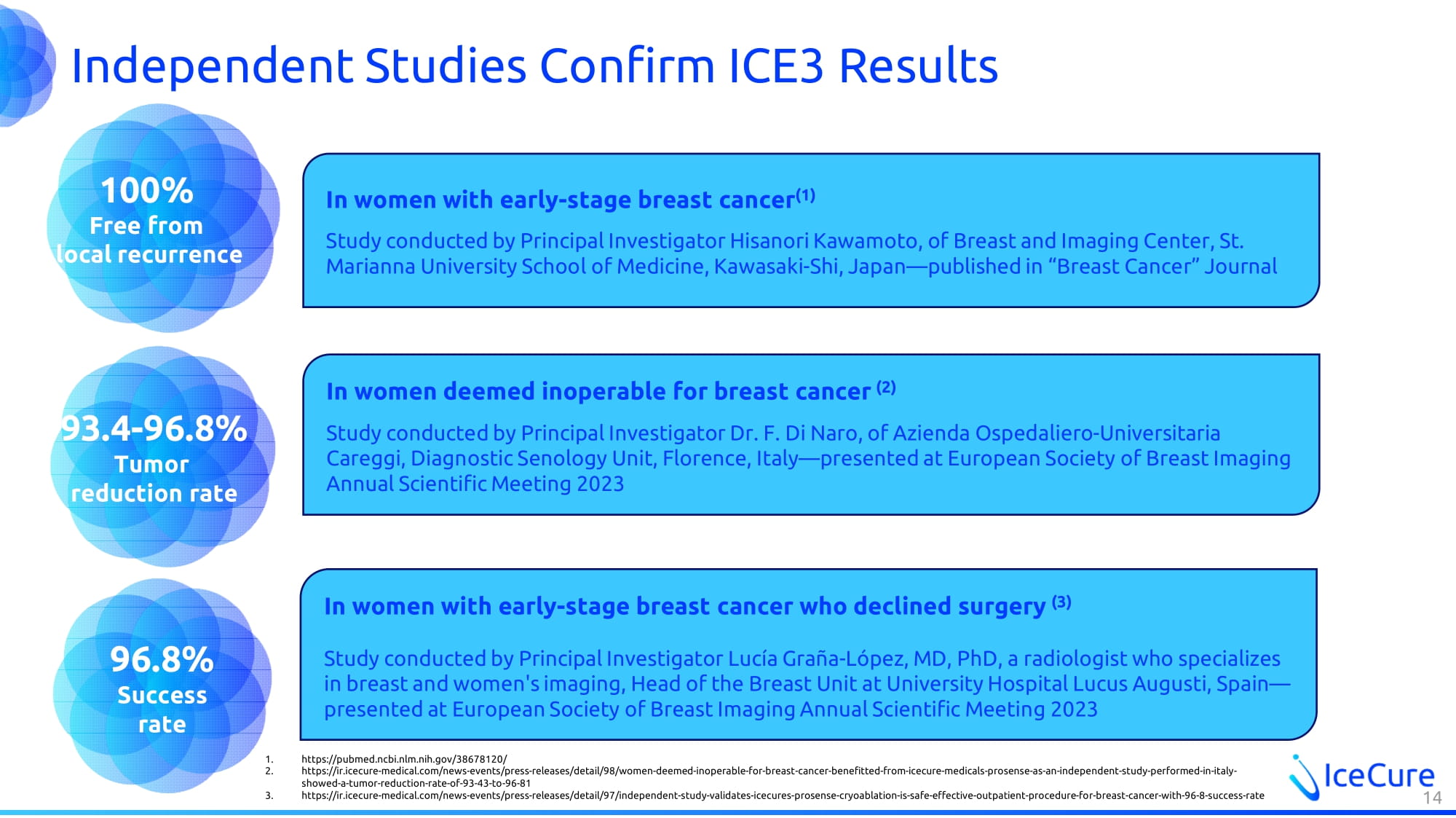
Independent Studies Confirm ICE3 Results 14 In women with early - stage breast cancer who declined surgery (3) Study conducted by Principal Investigator Lucía Graña - López, MD, PhD, a radiologist who specializes in breast and women's imaging, Head of the Breast Unit at University Hospital Lucus Augusti , Spain — presented at European Society of Breast Imaging Annual Scientific Meeting 2023 96.8% Success rate In women deemed inoperable for breast cancer (2) Study conducted by Principal Investigator Dr. F. Di Naro, of Azienda Ospedaliero - Universitaria Careggi, Diagnostic Senology Unit, Florence, Italy — presented at European Society of Breast Imaging Annual Scientific Meeting 2023 100% Free from local recurrence 93.4 - 96.8% Tumor reduction rate In women with early - stage breast cancer (1) Study conducted by Principal Investigator Hisanori Kawamoto, of Breast and Imaging Center , St. Marianna University School of Medicine, Kawasaki - Shi, Japan — published in “Breast Cancer” Journal 1. https://pubmed.ncbi.nlm.nih.gov/38678120/ 2. https://ir.icecure - medical.com/news - events/press - releases/detail/98/women - deemed - inoperable - for - breast - cancer - benefitted - from - ic ecure - medicals - prosense - as - an - independent - study - performed - in - italy - showed - a - tumor - reduction - rate - of - 93 - 43 - to - 96 - 81 3. https://ir.icecure - medical.com/news - events/press - releases/detail/97/independent - study - validates - icecures - prosense - cryoablation - i s - safe - effective - outpatient - procedure - for - breast - cancer - with - 96 - 8 - success - rate
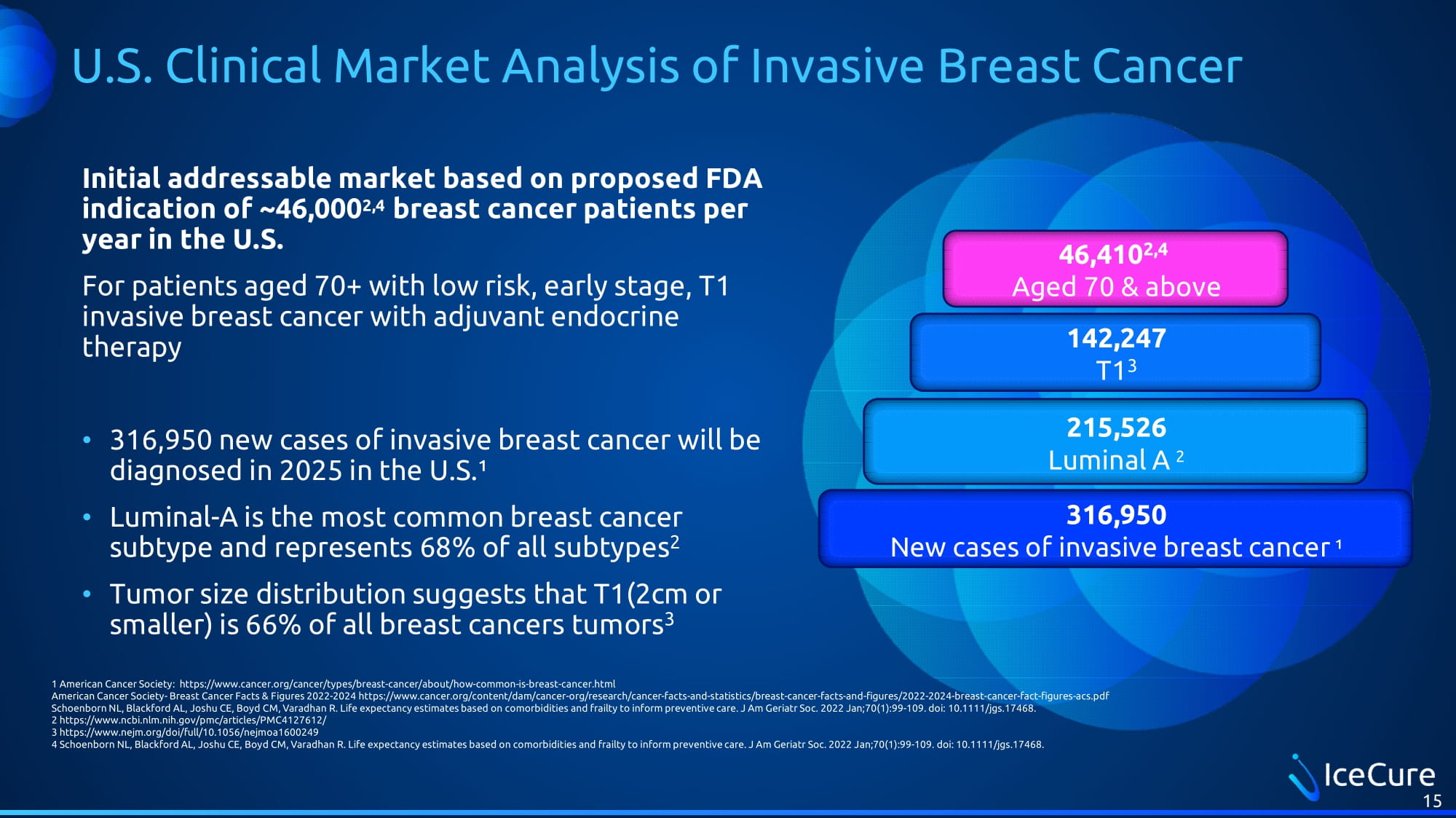
U.S. Clinical Market Analysis of Invasive Breast Cancer Initial addressable market based on proposed FDA indication of ~46,000 2,4 breast cancer patients per year in the U.S. For patients aged 70+ with low risk, early stage, T1 invasive breast cancer with adjuvant endocrine therapy • 316,950 new cases of invasive breast cancer will be diagnosed in 2025 in the U.S.¹ • Luminal - A is the most common breast cancer subtype and represents 68% of all subtypes 2 • Tumor size distribution suggests that T1(2cm or smaller) is 66% of all breast cancers tumors 3 15 316,950 New cases of invasive breast cancer ¹ 215,526 Luminal A 2 142,247 T1 3 46,410 2,4 Aged 70 & above 1 American Cancer Society: https://www.cancer.org/cancer/types/breast - cancer/about/how - common - is - breast - cancer.html American Cancer Society - Breast Cancer Facts & Figures 2022 - 2024 https://www.cancer.org/content/dam/cancer - org/research/cancer - f acts - and - statistics/breast - cancer - facts - and - figures/2022 - 2024 - breast - cancer - fact - figures - acs.pdf Schoenborn NL, Blackford AL, Joshu CE, Boyd CM, Varadhan R. Life expectancy estimates based on comorbidities and frailty to i nfo rm preventive care. J Am Geriatr Soc. 2022 Jan;70(1):99 - 109. doi : 10.1111/jgs. 17468. 2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4127612/ 3 https://www.nejm.org/doi/full/10.1056/nejmoa1600249 4 Schoenborn NL, Blackford AL, Joshu CE, Boyd CM, Varadhan R. Life expectancy estimates based on comorbidities and frailty to in form preventive care. J Am Geriatr Soc. 2022 Jan;70(1):99 - 109. doi : 10.1111/jgs.17468.

U.S. Breast Cancer Go - To - Market Strategy • FDA Decision Expected after the CDRH approves IceCure’s Post - Market Study Plan • IceCure has delivered a post - market study plan for approval which will include approximately 400 patients aged 70+ across 25 sites • Marketing & Distribution Ready • Direct sales to physicians, clinics, hospitals led by VP of North American Sales, Account Managers, and Clinical Experts • Developing breast cancer cryoablation treatment guidelines and courses with professional medical societies for breast surgeons ( ASBrS ) and radiologists (SBI, SIO, SIR) • Exploring strategic partnerships • Economics & Reimbursement • CMS assigned CPT Category III** at approximately $3,800 for the facility fee alone • Post - market study procedures eligible for reimbursement • Additional coverage, including physician fee, is expected upon establishment of the permanent CPT Category I code 16 **CPT or Current Procedural Terminology is a medical code used by physicians, health insurance companies and accreditation or gan izations for reimbursement CPT Category I: The largest body of codes, consisting of those commonly used by providers to report their services and procedures. CPT® Category III: Temporary codes used to report emerging and experimental services and procedures. Source: https://www.aapc.com/resources/medical - coding/cpt.aspx#:~:text=CPT%C2%AE%20Category%20I%3A%20The,and%20experimental%20services%2 0and%20procedures ; Establishment of CPT Category I is conditioned on factors including the Company's receipt of FDA marketing authorization of ProS ense® for breast cancer

Breast Cancer - Terumo Japan Agreement For >6 years ProSense ® has been sold through a Private Import License - now leveraging an agreement with Terumo to expand distribution and acquire PMDA approval Exclusive strategic distribution agreement with Terumo to accelerate commercialization of ProSense® in Japan $ 29.02 B 1 market cap $ 6.36 B 2 annual revenue 91,916 new breast cancer cases in Japan in 2022 3 • Total proceeds of $13.2M for the initial term x $ 5M for initial order and milestone - based payments x $ 4M received • Key terms: x Exclusive distribution of ProSense ® for breast cancer in Japan for 5 years post regulatory approval in Japan x Responsible for Japanese regulatory and reimbursement approvals x Terumo is expected to submit the request for breast cancer clearance to the Pharmaceuticals and Medical Devices Agency (PMDA) in H2 2025 1 As of June 5 2025; https://finance.yahoo.com/quote/4543.T/?guccounter=1 2 (TTM 3/31/2025); https://finance.yahoo.com/quote/4543.T/financials/ 3 392 - japan - fact - sheet.pdf 17
Interventional Radiology 18
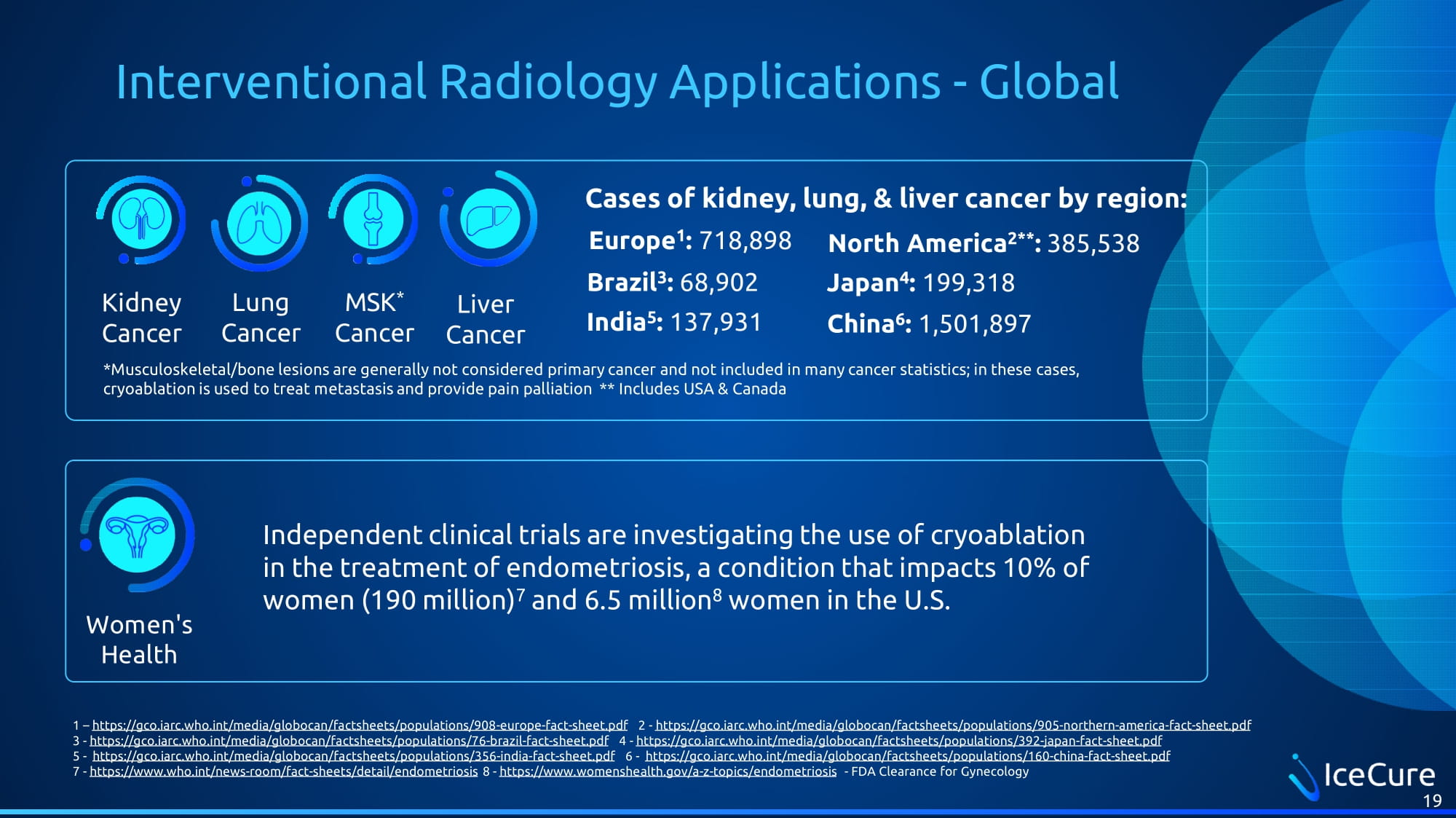
Interventional Radiology Applications - Global Kidney Cancer Lung Cancer MSK * Cancer Liver Cancer Europe 1 : 718,898 Women's Health 1 – https://gco.iarc.who.int/media/globocan/factsheets/populations/908 - europe - fact - sheet.pdf 2 - https://gco.iarc.who.int/media/globocan/factsheets/populations/905 - northern - america - fact - sheet.pdf 3 - https://gco.iarc.who.int/media/globocan/factsheets/populations/76 - brazil - fact - sheet.pdf 4 - https://gco.iarc.who.int/media/globocan/factsheets/populations/392 - japan - fact - sheet.pdf 5 - https://gco.iarc.who.int/media/globocan/factsheets/populations/356 - india - fact - sheet.pdf 6 - https://gco.iarc.who.int/media/globocan/factsheets/populations/160 - china - fact - sheet.pdf 7 - https://www.who.int/news - room/fact - sheets/detail/endometriosis 8 - https://www.womenshealth.gov/a - z - topics/endometriosis - FDA Clearance for Gynecology 19 Cases of kidney, lung, & liver cancer by region: Brazil 3 : 68,902 India 5 : 137,931 Japan 4 : 199,318 China 6 : 1,501,897 North America 2** : 385,538 Independent clinical trials are investigating the use of cryoablation in the treatment of endometriosis, a condition that impacts 10% of women (190 million) 7 and 6.5 million 8 women in the U.S. *Musculoskeletal/bone lesions are generally not considered primary cancer and not included in many cancer statistics; in thes e c ases, cryoablation is used to treat metastasis and provide pain palliation ** Includes USA & Canada

Kidney Cancer 88.7% recurrence - free rate Endometriosis 92.8% avoid secondary surgery IceCure ’ s ICESECRET ProSense® Trial Interim Results: • Highly effective for kidney tumors < 3 cm and a safe procedure for kidney tumors < 5 cm in people ineligible for surgery • 88.7 % were recurrence free at mean follow - up of period of 3.4 years Independent Study with ProSense® • 92 % recurrence free at mean follow - up of period of 22.2 months • 100 % secondary control rate when recurrent lesions are cryoablated Independent Clinical Trial in Japan with ProSense®: • No recurrence in patients with tumor size up to 1.2 cm • 4% recurrence in patients with tumor size between 1.3 – 1.7cm • 33% recurrence in patients with tumor size larger than 1.8 cm Lung Cancer 77% - 100% recurrence - free rate ProSense® One of Two Systems Used in Independent Study: • Efficacy rate in avoiding secondary surgery was 92.8% per patient and 93.6% per nodule treated • Median pain - free survival rates were 93.75% at 6 months and 82.72% after 12 months, 24 months, and 36 months collectively Interventional Radiology: Expanding Product Line Clinical data demonstrates ProSense ® ’s impact on various other indications 20

IceCure ’ s Global Reach Available directly or through distributors in the following countries EMEA – France, Germany, Italy, Spain, Poland, Romania, Hungary, Turkey, South Africa, Israel Asia – China, Hong Kong, Japan, India LATAM – Brazil, Costa Rica North America – USA & Canada 21
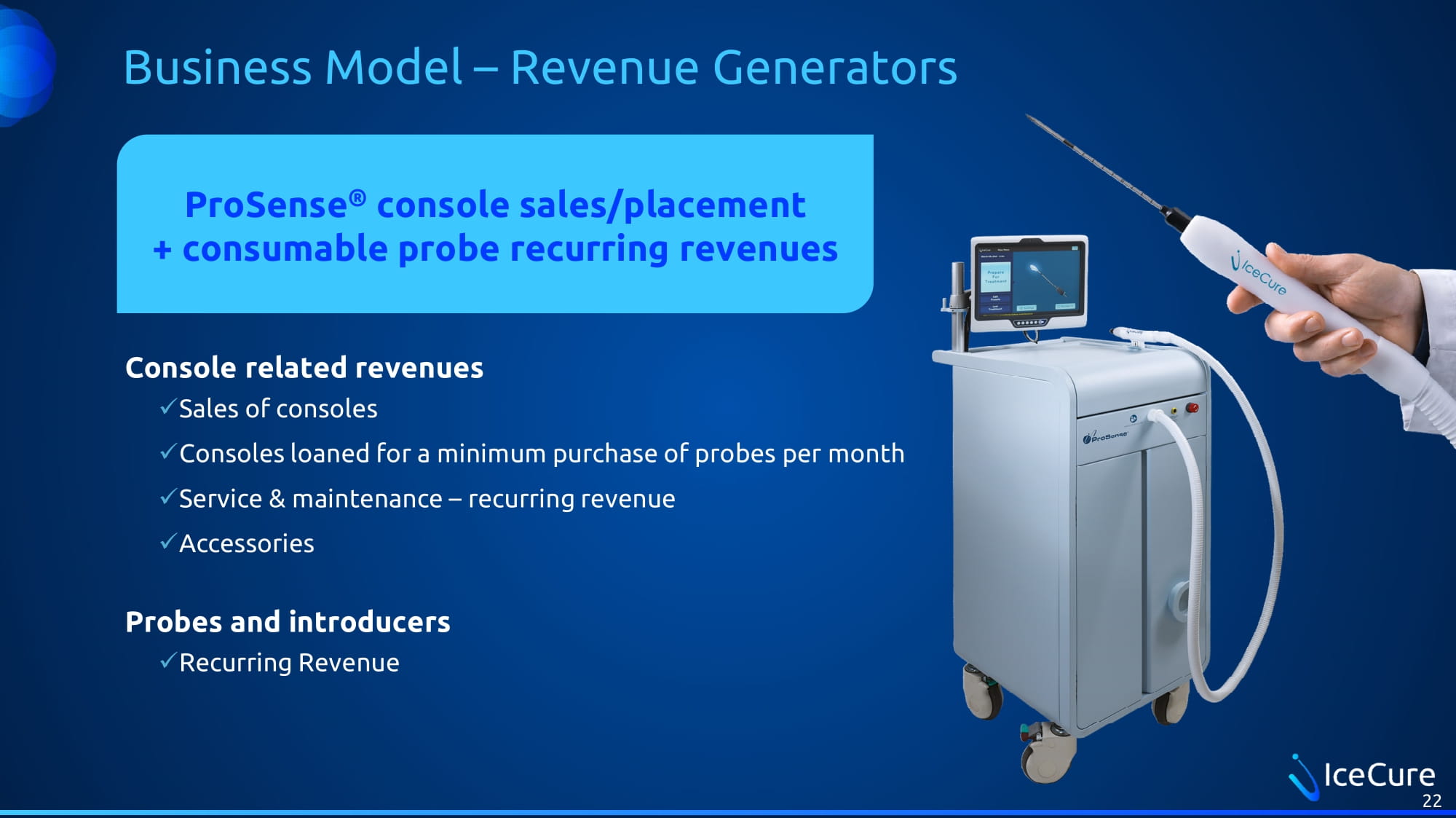
Business Model – Revenue Generators Console related revenues x Sales of consoles x Consoles loaned for a minimum purchase of probes per month x Service & maintenance – recurring revenue x Accessories Probes and introducers x Recurring Revenue ProSense ® console sales/placement + consumable probe recurring revenues 22

Upcoming Milestones and Strategy • FDA Decision Expected after the FDA’s Center for Devices and Radiological Health (CDRH) approves IceCure’s Post - Market Study Plan • Terumo, IceCure's distributor in Japan, is expected to submit the request for breast cancer clearance to Japan's Pharmaceuticals and Medical Devices Agency in H2 2025 • Greater market traction expected in the rest of world, based on positive U.S. ICE3 results data reported • More publications expected from ongoing independent studies of ProSense® worldwide in 2025 • Increasing direct sales of ProSense® systems and disposable probes in U.S. led by VP of Sales North America and U.S. team • Growing number of distribution partnerships to drive sales in rest of world with numerous recent regulatory approvals • Next generation XSense Cryoablation System received FDA 510(K) clearance, soft launch expected Q1 2026; XSense commercialization may lead to use for new clinical indications ProSense ® gaining global recognition as the leading cryoablation system for minimally invasive procedures in a $2.4 billion tumor ablation market 23
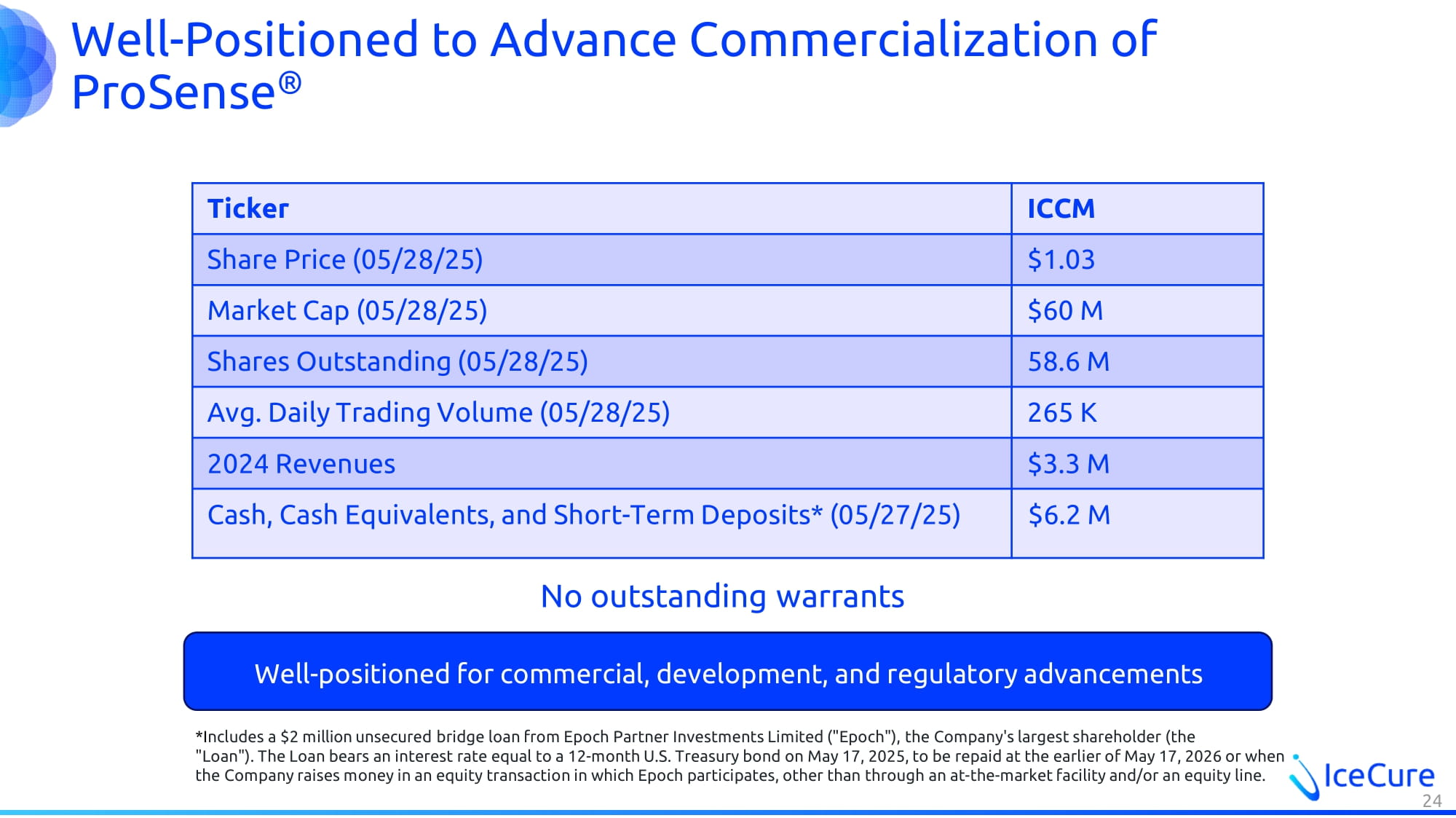
Well - Positioned to Advance Commercialization of ProSense ® ICCM Ticker $1.03 Share Price (05/28/25) $60 M Market Cap (05/28/25) 58.6 M Shares Outstanding (05/28/25) 265 K Avg. Daily Trading Volume (05/28/25) $3.3 M 2024 Revenues $6.2 M Cash, Cash Equivalents, and Short - Term Deposits* (05/27/25) 24 Well - positioned for commercial, development, and regulatory advancements No outstanding warrants * I ncludes a $ 2 million unsecured bridge loan from Epoch Partner Investments Limited ("Epoch"), the Company's largest shareholder (the "Loan"). The Loan bears an interest rate equal to a 12 - month U.S. Treasury bond on May 17 , 2025 , to be repaid at the earlier of May 17 , 2026 or when the Company raises money in an equity transaction in which Epoch participates, other than through an at - the - market facility and/ or an equity line.

Proven Leadership Team Ronen Tsimerman, CFO and COO Nearly 20 years’ experience as a CFO of public and private companies Ron Mayron, Chairman of the Board Served for 20 years in several positions at Teva including as VP – Israel & Africa & CEO of Teva Israel Shay Levav, VP Clinical, Regulatory & QA Nearly 20 years’ experience in regulatory and quality assurance in the healthcare sector Tlalit Bussi Tel - Tzure , VP Global Business Development & Marketing Over 15 years’ experience in Sales, Biz Dev & Marketing in medical devices Naum Muchnick, VP R&D Nearly 20 years of experience in medical device design, engineering, and operations, including over 13 years with GE UltraSound Eyal Shamir, CEO Over 15 years as CEO of medical device companies (B - Cure Laser, Hanita Lenses etc.) Merav Nir Dotan, VP Human Resources Over 20 years of experience in human resources and organizational management Galit Bar Malik, VP Operations & Service Over 20 years of experience medical device operations Shad Good, VP Sales North America Nearly 20 years of medical device sales and leadership with experience in minimally invasive breast diagnostic and therapeutic systems 25

Thank You! 26 Eyal Shamir, CEO Ronen Tsimerman – CFO/COO E: investors@icecure - medical.com T: + 972 - 4 - 623 - 0333

Appendix Additional Resources 27
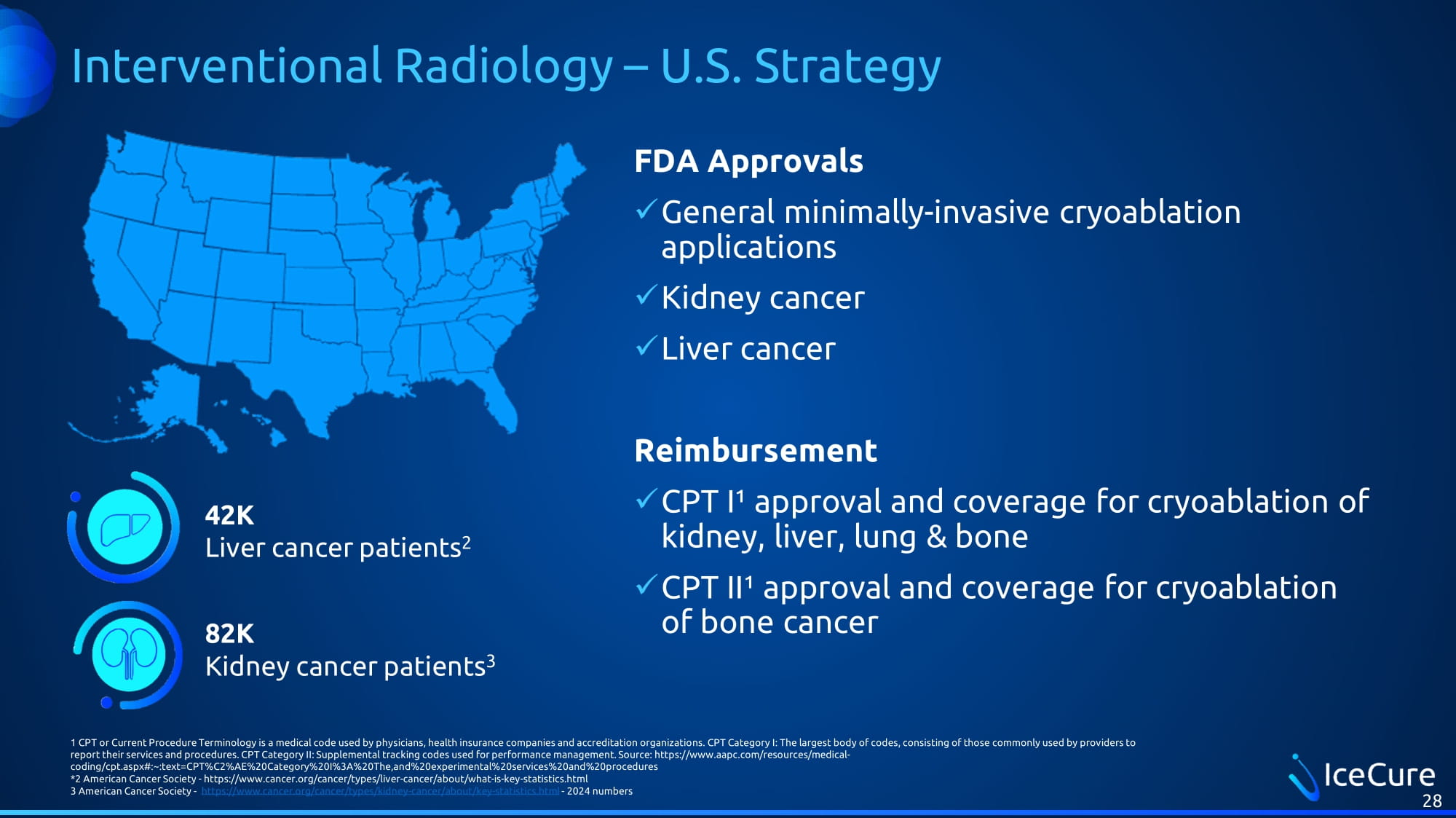
Interventional Radiology – U.S. Strategy FDA Approvals x General minimally - invasive cryoablation applications x Kidney cancer x Liver cancer Reimbursement x CPT I¹ approval and coverage for cryoablation of kidney, liver, lung & bone x CPT II¹ approval and coverage for cryoablation of bone cancer 28 1 CPT or Current Procedure Terminology is a medical code used by physicians, health insurance companies and accreditation organ iza tions. CPT Category I: The largest body of codes, consisting of those commonly used by providers to report their services and procedures. CPT Category II: Supplemental tracking codes used for performance management. Source: h ttp s://www.aapc.com/resources/medical - coding/cpt.aspx#:~:text=CPT%C 2 %AE% 20 Category% 20 I% 3 A% 20 The,and% 20 experimental% 20 services% 20 and% 20 procedures * 2 American Cancer Society - https://www.cancer.org/cancer/types/liver - cancer/about/what - is - key - statistics.html 3 American Cancer Society - https://www.cancer.org/cancer/types/kidney - cancer/about/key - statistics.html - 2024 numbers 42K Liver cancer patients 2 82K Kidney cancer patients 3
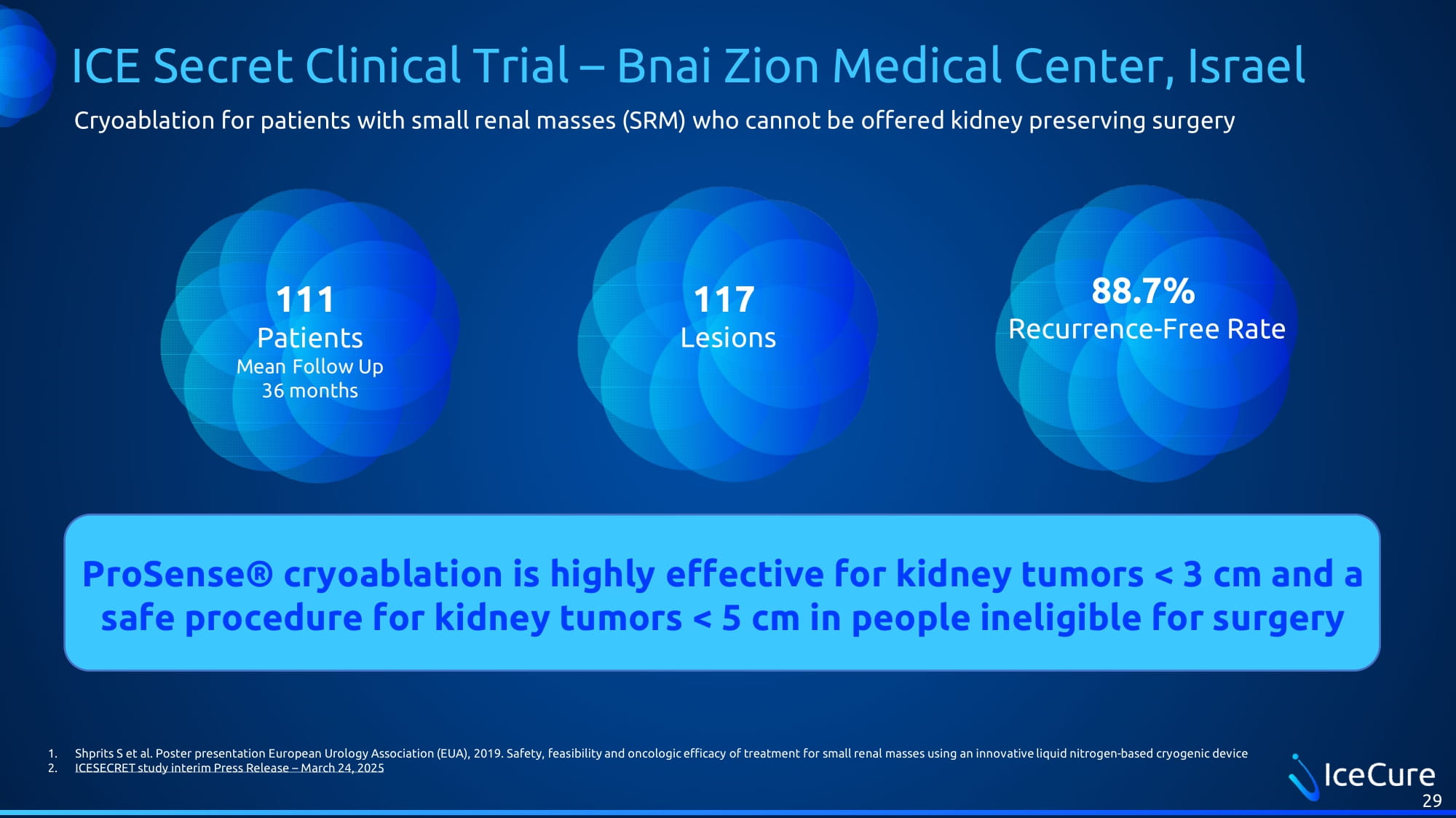
ICE Secret Clinical Trial – Bnai Zion Medical Center, Israel 29 111 Patients Mean Follow Up 36 months 88.7% Recurrence - Free Rate ProSense® cryoablation is highly effective for kidney tumors < 3 cm and a safe procedure for kidney tumors < 5 cm in people ineligible for surgery 1. Shprits S et al. Poster presentation European Urology Association (EUA), 2019 . Safety, feasibility and oncologic efficacy of treatment for small renal masses using an innovative liquid nitrogen - based cryog enic device 2. ICESECRET study interim Press Release – March 24 , 2025 Cryoablation for patients with small renal masses (SRM) who cannot be offered kidney preserving surgery 117 Lesions
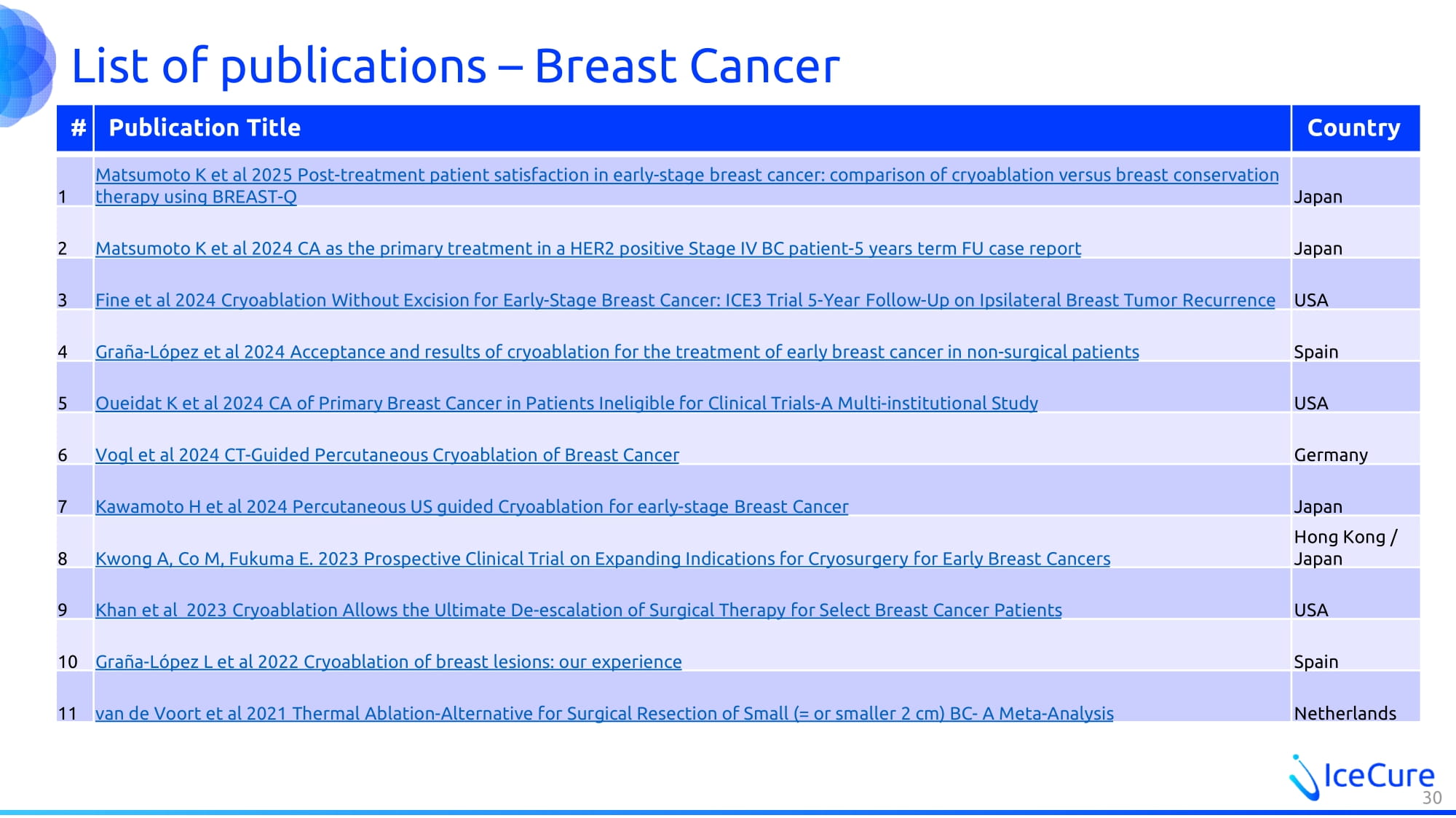
List of publications – Breast Cancer Country Publication Title # Japan Matsumoto K et al 2025 Post - treatment patient satisfaction in early - stage breast cancer: comparison of cryoablation versus breas t conservation therapy using BREAST - Q 1 Japan Matsumoto K et al 2024 CA as the primary treatment in a HER2 positive Stage IV BC patient - 5 years term FU case report 2 USA Fine et al 2024 Cryoablation Without Excision for Early - Stage Breast Cancer: ICE3 Trial 5 - Year Follow - Up on Ipsilateral Breast Tumor Recurrence 3 Spain Graña - López et al 2024 Acceptance and results of cryoablation for the treatment of early breast cancer in non - surgical patients 4 USA Oueidat K et al 2024 CA of Primary Breast Cancer in Patients Ineligible for Clinical Trials - A Multi - institutional Study 5 Germany Vogl et al 2024 CT - Guided Percutaneous Cryoablation of Breast Cancer 6 Japan Kawamoto H et al 2024 Percutaneous US guided Cryoablation for early - stage Breast Cancer 7 Hong Kong / Japan Kwong A, Co M, Fukuma E. 2023 Prospective Clinical Trial on Expanding Indications for Cryosurgery for Early Breast Cancers 8 USA Khan et al 2023 Cryoablation Allows the Ultimate De - escalation of Surgical Therapy for Select Breast Cancer Patients 9 Spain Graña - López L et al 2022 Cryoablation of breast lesions: our experience 10 Netherlands van de Voort et al 2021 Thermal Ablation - Alternative for Surgical Resection of Small (= or smaller 2 cm) BC - A Meta - Analysis 11 30
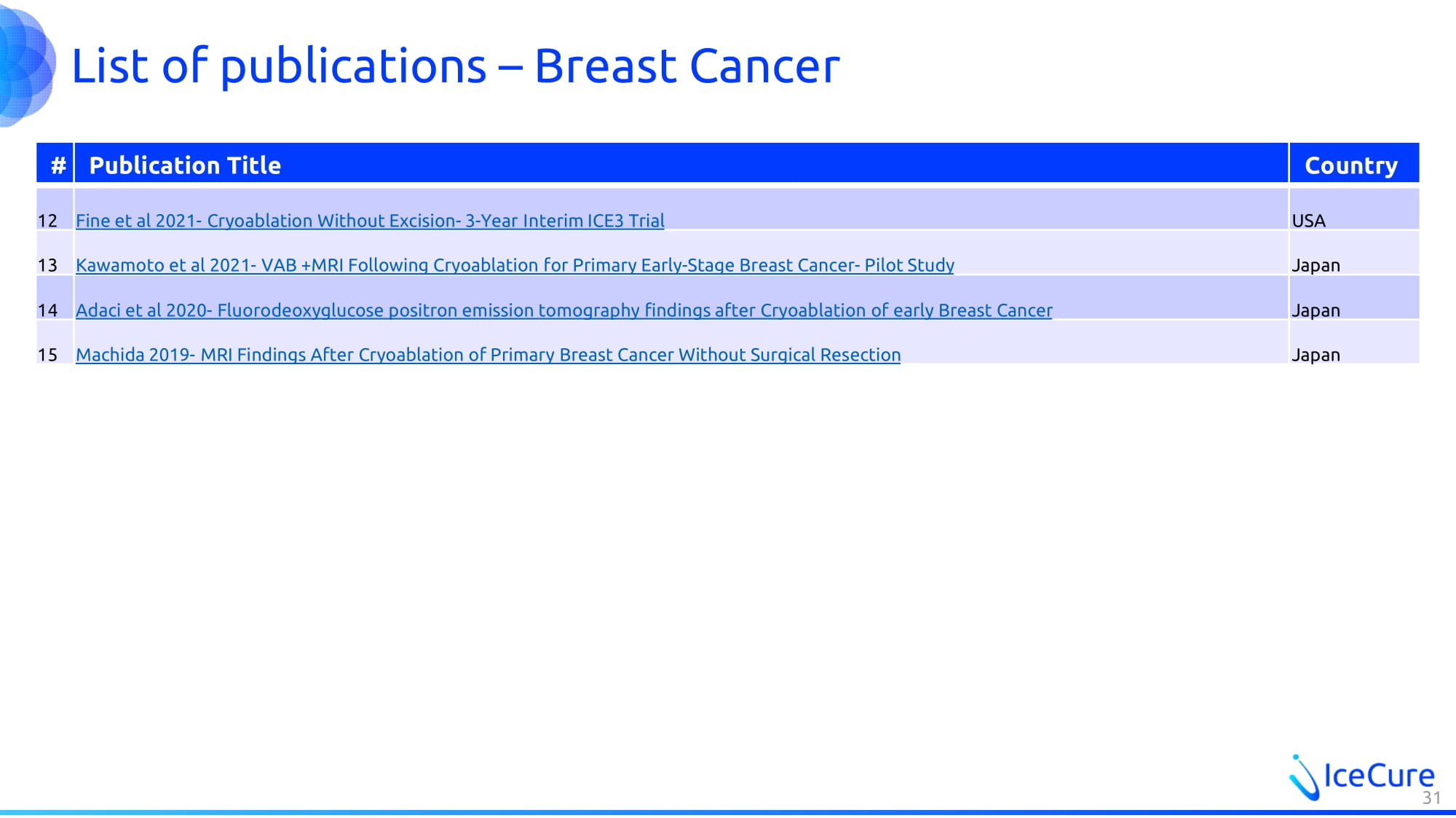
List of publications – Breast Cancer Country Publication Title # USA Fine et al 2021 - Cryoablation Without Excision - 3 - Year Interim ICE3 Trial 12 Japan Kawamoto et al 2021 - VAB +MRI Following Cryoablation for Primary Early - Stage Breast Cancer - Pilot Study 13 Japan Adaci et al 2020 - Fluorodeoxyglucose positron emission tomography findings after Cryoablation of early Breast Cancer 14 Japan Machida 2019 - MRI Findings After Cryoablation of Primary Breast Cancer Without Surgical Resection 15 31
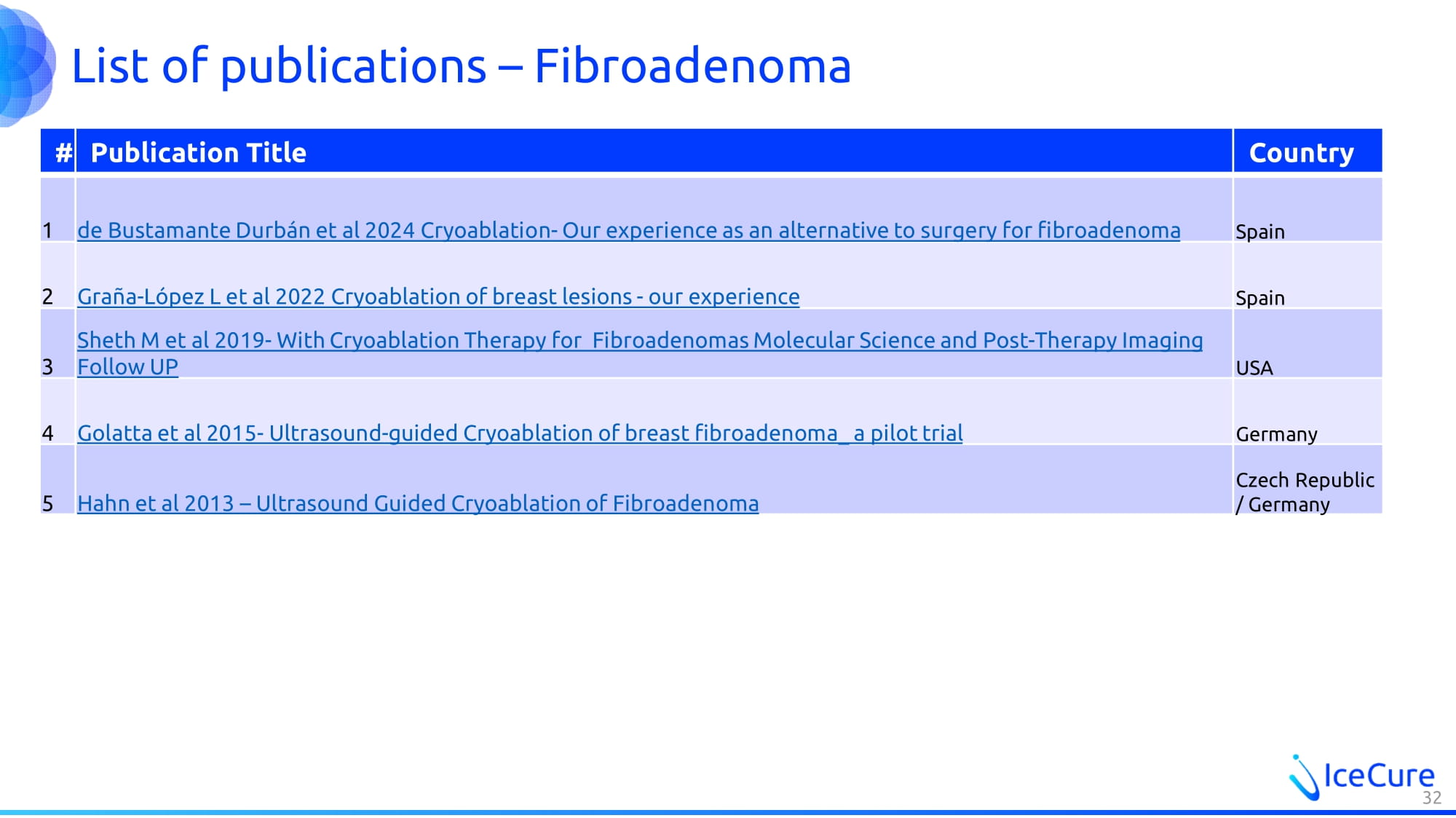
List of publications – Fibroadenoma Country Publication Title # Spain de Bustamante Durbán et al 2024 Cryoablation - Our experience as an alternative to surgery for fibroadenoma 1 Spain Graña - López L et al 2022 Cryoablation of breast lesions - our experience 2 USA Sheth M et al 2019 - With Cryoablation Therapy for Fibroadenomas Molecular Science and Post - Therapy Imaging Follow UP 3 Germany Golatta et al 2015 - Ultrasound - guided Cryoablation of breast fibroadenoma_ a pilot trial 4 Czech Republic / Germany Hahn et al 2013 – Ultrasound Guided Cryoablation of Fibroadenoma 5 32
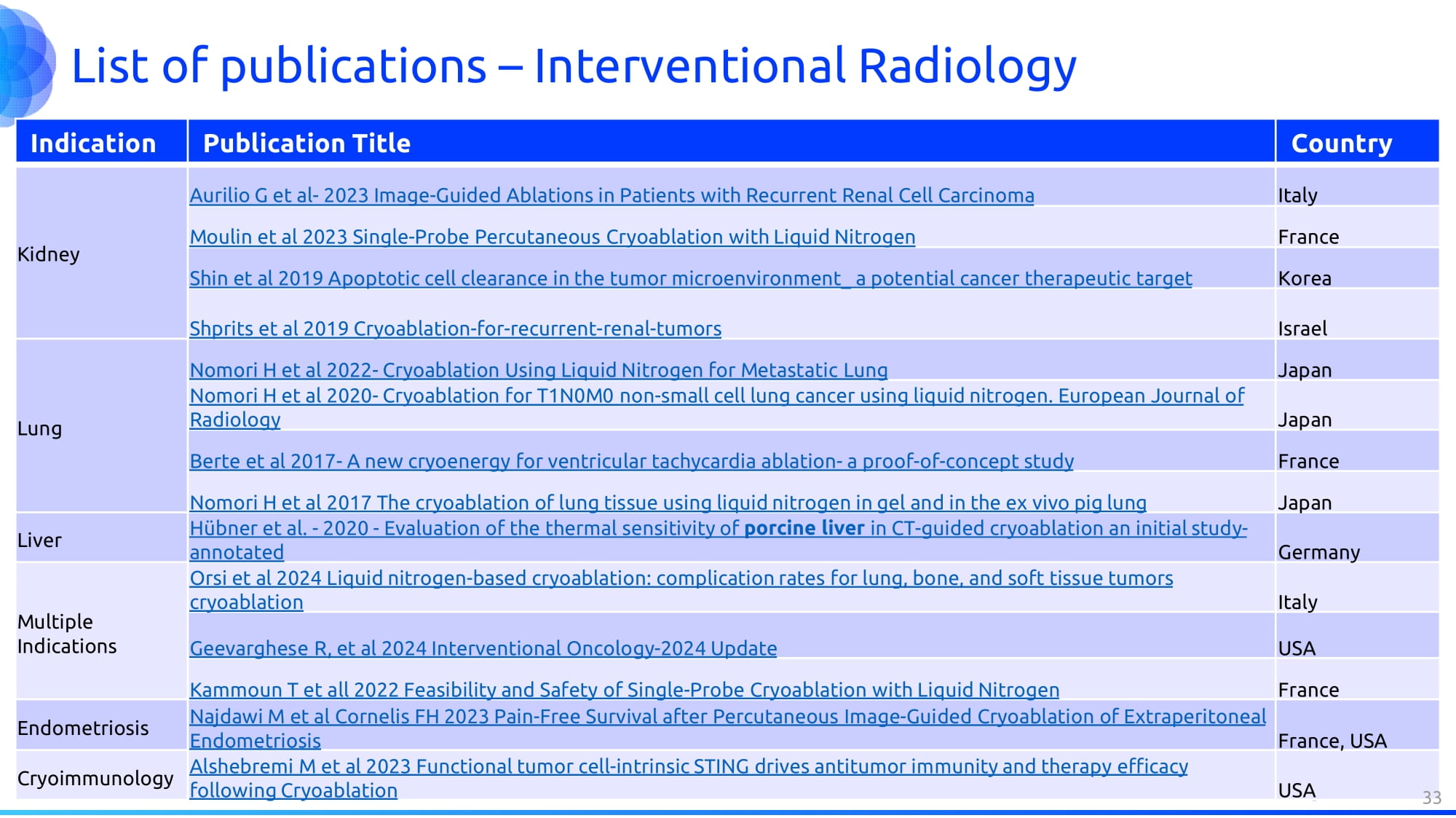
List of publications – Interventional Radiology Country Publication Title Indication Italy Aurilio G et al - 2023 Image - Guided Ablations in Patients with Recurrent Renal Cell Carcinoma Kidney France Moulin et al 2023 Single - Probe Percutaneous Cryoablation with Liquid Nitrogen Korea Shin et al 2019 Apoptotic cell clearance in the tumor microenvironment_ a potential cancer therapeutic target Israel Shprits et al 2019 Cryoablation - for - recurrent - renal - tumors Japan Nomori H et al 2022 - Cryoablation Using Liquid Nitrogen for Metastatic Lung Lung Japan Nomori H et al 2020 - Cryoablation for T1N0M0 non - small cell lung cancer using liquid nitrogen. European Journal of Radiology France Berte et al 2017 - A new cryoenergy for ventricular tachycardia ablation - a proof - of - concept study Japan Nomori H et al 2017 The cryoablation of lung tissue using liquid nitrogen in gel and in the ex vivo pig lung Germany Hübner et al. - 2020 - Evaluation of the thermal sensitivity of porcine liver in CT - guided cryoablation an initial study - annotated Liver Italy Orsi et al 2024 Liquid nitrogen - based cryoablation: complication rates for lung, bone, and soft tissue tumors cryoablation Multiple Indications USA Geevarghese R, et al 2024 Interventional Oncology - 2024 Update France Kammoun T et all 2022 Feasibility and Safety of Single - Probe Cryoablation with Liquid Nitrogen France, USA Najdawi M et al Cornelis FH 2023 Pain - Free Survival after Percutaneous Image - Guided Cryoablation of Extraperitoneal Endometriosis Endometriosis USA Alshebremi M et al 2023 Functional tumor cell - intrinsic STING drives antitumor immunity and therapy efficacy following Cryoablation Cryoimmunology 33